Membrane insertion exacerbates the α-Synuclein-Cu(II) dopamine oxidase activity: Metallothionein-3 targets and silences all α-synuclein-Cu(II) complexes
- PMID: 32712192
- PMCID: PMC7484060
- DOI: 10.1016/j.freeradbiomed.2020.07.006
Membrane insertion exacerbates the α-Synuclein-Cu(II) dopamine oxidase activity: Metallothionein-3 targets and silences all α-synuclein-Cu(II) complexes
Abstract
Copper binding to α-synuclein (α-Syn), the major component of intracellular Lewy body inclusions in substantia nigra dopaminergic neurons, potentiate its toxic redox-reactivity and plays a detrimental role in the etiology of Parkinson disease (PD). Soluble α-synuclein-Cu(II) complexes possess dopamine oxidase activity and catalyze ROS production in the presence of biological reducing agents via Cu(II)/Cu(I) redox cycling. These metal-centered redox reactivities harmfully promote the oxidation and oligomerization of α-Syn. While this chemistry has been investigated on recombinantly expressed soluble α-Syn, in vivo, α-Syn is acetylated at its N-terminus and is present in equilibrium between soluble and membrane-bound forms. This post-translational modification and membrane-binding alter the Cu(II) coordination environment and binding modes and are expected to affect the α-Syn-Cu(II) reactivity. In this work, we first investigated the reactivity of acetylated and membrane-bound complexes, and subsequently addressed whether the brain metalloprotein Zn7-metallothionein-3 (Zn7MT-3) possesses a multifaceted-role in targeting these aberrant copper interactions and consequent reactivity. Through biochemical characterization of the reactivity of the non-acetylated/N-terminally acetylated soluble or membrane-bound α-Syn-Cu(II) complexes towards dopamine, oxygen, and ascorbate, we reveal that membrane insertion dramatically exacerbates the catechol oxidase-like reactivity of α-Syn-Cu(II) as a result of a change in the Cu(II) coordination environment, thereby potentiating its toxicity. Moreover, we show that Zn7MT-3 can efficiently target all α-Syn-Cu(II) complexes through Cu(II) removal, preventing their deleterious redox activities. We demonstrate that the Cu(II) reduction by the thiolate ligands of Zn7MT-3 and the formation of Cu(I)4Zn4MT-3 featuring an unusual redox-inert Cu(I)4-thiolate cluster is the molecular mechanism responsible for the protective effect exerted by MT-3 towards α-Syn-Cu(II). This work provides the molecular basis for new therapeutic interventions to control the deleterious bioinorganic chemistry of α-Syn-Cu(II).
Keywords: Alpha-synuclein; Copper dysregulation; Dopamine oxidation; Metallothionein-3; Parkinson's disease; Reactive oxygen species.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



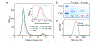

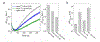



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

