Valproic acid upregulates the expression of the p75NTR/sortilin receptor complex to induce neuronal apoptosis
- PMID: 32712736
- PMCID: PMC7527367
- DOI: 10.1007/s10495-020-01626-0
Valproic acid upregulates the expression of the p75NTR/sortilin receptor complex to induce neuronal apoptosis
Abstract
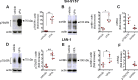
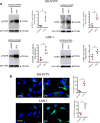
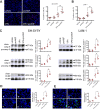
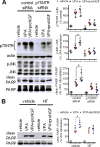
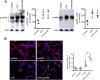
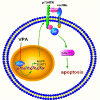
The antiepileptic and mood stabilizer agent valproic acid (VPA) has been shown to exert anti-tumour effects and to cause neuronal damage in the developing brain through mechanisms not completely understood. In the present study we show that prolonged exposure of SH-SY5Y and LAN-1 human neuroblastoma cells to clinically relevant concentrations of VPA caused a marked induction of the protein and transcript levels of the common neurotrophin receptor p75NTR and its co-receptor sortilin, two promoters of apoptotic cell death in response to proneurotrophins. VPA induction of p75NTR and sortilin was associated with an increase in plasma membrane expression of the receptor proteins and was mimicked by cell treatment with several histone deacetylase (HDAC) inhibitors. VPA and HDAC1 knockdown decreased the level of EZH2, a core component of the polycomb repressive complex 2, and upregulated the transcription factor CASZ1, a positive regulator of p75NTR. CASZ1 knockdown attenuated VPA-induced p75NTR overexpression. Cell treatment with VPA favoured proNGF-induced p75NTR/sortilin interaction and the exposure to proNGF enhanced JNK activation and apoptotic cell death elicited by VPA. Depletion of p75NTR or addition of the sortilin agonist neurotensin to block proNGF/sortilin interaction reduced the apoptotic response to VPA and proNGF. Exposure of mouse cerebellar granule cells to VPA upregulated p75NTR and sortilin and induced apoptosis which was enhanced by proNGF. These results indicate that VPA upregulates p75NTR apoptotic cell signalling through an epigenetic mechanism involving HDAC inhibition and suggest that this effect may contribute to the anti-neuroblastoma and neurotoxic effects of VPA.
Keywords: Apoptosis; Histone deacetylase inhibitors; Human neuroblastoma cells; Mouse cerebellar granule cells; Sortilin; p75NTR.
Figures




References
-
- Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. - PubMed
-
- Henry TR. The history of valproate in clinical neuroscience. Psychopharmacology Bull. 2003;37:5–16. - PubMed
-
- Duenas-Gonzales A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug; preclinical, clinical and transcriptional effects on solid tumors. Cancer Ther Rev. 2008;34:206–222. - PubMed
-
- Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol Therap. 2001;1:21–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

