Efficacy and safety of an innovative prolonged-release combination drug in patients with distal renal tubular acidosis: an open-label comparative trial versus standard of care treatments
- PMID: 32712761
- PMCID: PMC7701073
- DOI: 10.1007/s00467-020-04693-2
Efficacy and safety of an innovative prolonged-release combination drug in patients with distal renal tubular acidosis: an open-label comparative trial versus standard of care treatments
Erratum in
-
Correction to: Efficacy and safety of an innovative prolonged-release combination drug in patients with distal renal tubular acidosis: an open-label comparative trial versus standard of care treatments.Pediatr Nephrol. 2021 Jan;36(1):215. doi: 10.1007/s00467-020-04776-0. Pediatr Nephrol. 2021. PMID: 32989611 Free PMC article.
Abstract
Background: Distal renal tubular acidosis (dRTA), due to impaired acid secretion in the urine, can lead to severe long-term consequences. Standard of care (SoC) oral alkalizers, requiring several daily intakes, are currently used to restore normal plasma bicarbonate levels. A new prolonged-release formulation, ADV7103, has been developed to achieve a sustained effect with an improved dosing scheme.
Methods: In a multicenter, open-label, non-inferiority trial (n = 37), patients with dRTA were switched from SoC to ADV7103. Mean plasma bicarbonate values and proportion of responders during steady state therapy with both treatments were compared, as were other blood and urine parameters, as well as acceptability, tolerability, and safety.
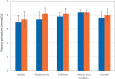
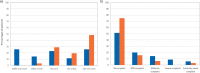
Results: When switching from SoC to ADV7103, the number of daily intakes was reduced from a median of three to twice daily. Mean plasma bicarbonate was increased and non-inferiority of ADV7103 was demonstrated (p < 0.0001, per protocol), as was statistical superiority (p = 0.0008, intention to treat [ITT]), and the response rate increased from 43 to 90% with ADV7103 (p < 0.001, ITT). Urine calcium/citrate ratio was reduced below the threshold for risk of lithogenesis with ADV7103 in 56% of previously non-responders with SoC (p = 0.021, ITT). Palatability was improved (difference [95% CI] of 25 [10.7, 39.2] mm) and gastrointestinal discomfort was reduced (difference [95% CI] of - 14.2 [- 25.9, - 2.6] mm) with ADV7103.
Conclusions: Plasma bicarbonate levels and response rate were significantly higher with ADV7103 than with SoC. Urine calcium/citrate ratio, palatability, and gastrointestinal safety were significantly improved, supporting the use of ADV7103 as first-line treatment for dRTA.
Trial registration: Registered as EudraCT 2013-002988-25 on the 1st July 2013 Graphical abstract.
Keywords: Gastrointestinal tolerability; Palatability; Plasma bicarbonate; Plasma potassium; dRTA.
Conflict of interest statement
C. Guittet, M.A. Manso-Silván, and L.A. Granier are employees of Advicenne and hold stock options or shares in the company. A. Castang is also an employee of Advicenne. C. Stylianou was paid (contract research) for his contribution to statistical analyses. A. Bertholet perceived support from Advicenne for traveling to meetings and/or funding for lectures.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

