Convalescent plasma for covid19 - How long should a donor be excluded from donation?
- PMID: 32713627
- PMCID: PMC7362863
- DOI: 10.1016/j.transci.2020.102873
Convalescent plasma for covid19 - How long should a donor be excluded from donation?
Conflict of interest statement
The authors declare no conflicts of interest
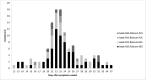
Figures
Comment on
-
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.Euro Surveill. 2020 Jan;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Euro Surveill. 2020. PMID: 31992387 Free PMC article.
References
-
- FDA – Food and Drug Administration. Investigational COVID-19 Convalescent Plasma, Guidance for Industry. Available www.URL: https://www.fda.gov/media/136798/download, accessed April 20th, 2020.
-
- 2020. European Commission - An EU Program of COVID-19 Convalescent Plasma Collection and Transfusion. Available www.URL: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs..., accessed April 20th. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical