Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review
- PMID: 32713784
- PMCID: PMC7369580
- DOI: 10.1016/j.pulmoe.2020.07.003
Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review
Abstract
Background: Tocilizumab is an IL-6 receptor-blocking agent proposed for the treatment of severe COVID-19. The aim of this systematic review was to describe the rationale for the use of tocilizumab for the treatment of COVID-19 and to summarize the available evidence regarding its efficacy and safety.
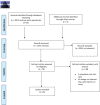
Methods: MEDLINE, PubMed, EMBASE, pre-print repositories (bioRxiv and medRxiv) and two trial Registries were searched for studies on the use of tocilizumab in COVID-19 or SARS-CoV-2 infection, viral pneumonia, and/or sepsis until 20th June 2020.
Results: We identified 3 indirect pre-clinical studies and 28 clinical studies including 5776 patients with COVID-19 (13 with a comparison group, 15 single-arm). To date, no randomized trials have been published. We retrieved no studies at low risk of bias. Forty-five ongoing studies were retrieved from trial registries.
Conclusions: There is insufficient evidence regarding the clinical efficacy and safety of tocilizumab in patients with COVID-19. Its use should be considered experimental, requiring ethical approval and clinical trial oversight.
Keywords: COVID-19; Coronavirus; Pneumonia; SARS-CoV-2; Tocilizumab.
Copyright © 2020 Sociedade Portuguesa de Pneumologia. Published by Elsevier España, S.L.U. All rights reserved.
Figures

Comment in
-
Interleukin-6 blockade with tocilizumab in COVID-19: Does it live up to its hype?Pulmonology. 2021 Jan-Feb;27(1):86-87. doi: 10.1016/j.pulmoe.2020.10.004. Epub 2020 Oct 21. Pulmonology. 2021. PMID: 33158786 Free PMC article. No abstract available.
-
Rationale and evidence on the use of tocilizumab in COVID-19: A systematic review. Authors' reply.Pulmonology. 2021 Jan-Feb;27(1):87-88. doi: 10.1016/j.pulmoe.2020.10.003. Epub 2020 Oct 21. Pulmonology. 2021. PMID: 33158787 Free PMC article. No abstract available.
-
Efficacy of tocilizumab in COVID-19: A review of the current evidence.Sci Prog. 2021 Jul-Sep;104(3):368504211030372. doi: 10.1177/00368504211030372. Sci Prog. 2021. PMID: 34236264 Free PMC article.
References
-
- WHO. COVID-19 Situation report – 139. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... 2020. [Accessed June 7, 2020].
-
- Johns Hopkins University & Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/map.html. 2020. [Accessed June 7, 2020].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
