Anti-inflammatory effects of powdered product of Bu Yang Huan Wu decoction: Possible role in protecting against Transient Focal Cerebral Ischemia
- PMID: 32714088
- PMCID: PMC7378667
- DOI: 10.7150/ijms.46581
Anti-inflammatory effects of powdered product of Bu Yang Huan Wu decoction: Possible role in protecting against Transient Focal Cerebral Ischemia
Abstract
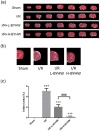
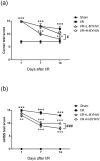
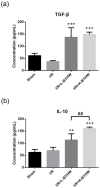
Bu Yang Huan Wu decoction (BYHW) is a traditional Chinese medicine (TCM) that consists of several herbs and has been used in patients with ischemic stroke for centuries. Although powdered formula of BYHW has widely been prescribed in clinic nowadays, evidence-based effectiveness and mechanism of action of BYHW powdered product in stroke remain to be characterized. Adult male Sprague-Dawley rats were subjected to middle cerebral artery occlusion (MCAO) for 90 min followed by reperfusion for 24 h (ischemia/reperfusion; I/R) or sham surgery. After I/R, the rats were then given low dose (0.5 g/kg) and high dose (2.5 g/kg) of BYHW or vehicle by oral gavage twice a day for seven consecutive days. The results showed that I/R induced obvious cerebral infarction and neurobehavioral defects, in parallel with histological aberrations and extensive signaling of proinflammatory cytokines, including tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), in the stroke model. Post-I/R treatment with BYHW powdered product significantly reduced the infarct area and ameliorated neurofunctional defects in a dose-dependent manner. The dose dependence was associated with TNF-α downregulation and interleukin-10 (IL-10) induction. In summary, the present findings demonstrated that BYHW powdered product exhibited therapeutic efficacy for experimental stroke and a higher dose treatment may strengthen the effectiveness via inflammatory modulation.
Keywords: Bu Yang Huan Wu decoction; Ischemic/reperfusion; Stroke; inflammation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

