Cell Death by Gallotannin Is Associated with Inhibition of the JAK/STAT Pathway in Human Colon Cancer Cells
- PMID: 32714471
- PMCID: PMC7378856
- DOI: 10.1016/j.curtheres.2020.100589
Cell Death by Gallotannin Is Associated with Inhibition of the JAK/STAT Pathway in Human Colon Cancer Cells
Abstract
Background: Gallotannin (GT) is a polyphenol that possesses interesting anticancer properties. However, the mechanisms underlying its antitumor effects have not been well defined.
Objective: This study was designed to clarify the mechanisms underlying GT antitumor effects in colon cancer cell lines.
Methods: Three isogenic HCT116 cell lines (p53+/+, p53-/-, and p21-/-) were treated with GT for different time points then Western blot, flow cytometry, and senescence analysis were performed to examine the effect of GT on Mitogen-activated protein kinase (MAPK) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) effectors, STAT3 downstream apoptotic targets, Sub-G1 phase, and programmed cell death induction. Transfection using Invitrogen Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, Waltham, Massachusetts) were used to identify the role of p53 and p21 in the p53-/- and p21-/- cell lines.
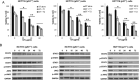
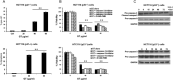
Results: Both low and high GT concentrations caused MAPKs activation marked by upregulation of extracellular signal-regulated kinase (p-ERK). The preincubation with the antioxidant Tiron (Sigma-Aldrich, St Louis, Missouri) showed that GT's antitumor effects were not mediated by reactive oxygen species. We then examined the effect of GT on the JAK/STAT pathway, which is known to be activated in colorectal cancer. GT totally inhibited the JAK/STAT pathway effectors JAK2, STAT1, and STAT3 and their downstream apoptotic regulators B-cell lymphoma-extra large (Bcl-xL) and c-Myc in all 3 cell lines. HCT116 cancer cells exhibited differential sensitivity to GT with p21-/- cells being the most sensitive and p53+/+ cells that express p21 protein being the least sensitive. In p53+/+ cells, GT induced senescence, whereas in p53-/- and p21-/- cells, GT induced apoptosis in a caspase independent manner marked by Poly(ADP-Ribose) Polymerase (PARP) cleavage, Bcl-2 downregulation, and upregulation of the Bcl-2 associated X (Bax) to B-cell lymphoma 2 (Bcl-2) ratio. In addition, the sub-G1 phase exceeded 50% in p21-/- cells.
Conclusions: Considered together, our results indicate that GT is potent inhibitor of the JAK/STAT pathway in colon cancer irrespective of the p53 and p21 status, which provides insights into its mechanism of anticancer activities and future potential for clinical translation. (Curr Ther Res Clin Exp. 2020; 81:XXX-XXX).
Keywords: Apoptosis; JAK/STAT; caspase; colon cancer; gallotannin; senescence.
© 2020 The Authors.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. - PubMed
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:104–117. - PubMed
-
- Umar A, Viner JL, Richmond E, Anderson WF, Hawk ET. Chemoprevention of colorectal carcinogenesis. International journal of clinical oncology. 2002;7:2–26. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

