The Endocrine System and Alcohol Drinking in Females
- PMID: 32714716
- PMCID: PMC7374925
- DOI: 10.35946/arcr.v40.2.02
The Endocrine System and Alcohol Drinking in Females
Abstract
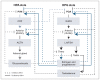
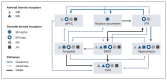
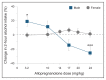
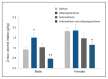
Sexually dimorphic effects of alcohol exposure throughout life have been documented in clinical and preclinical studies. In the past, rates of alcohol use disorder (AUD) were higher in men than in women, but over the past 10 years, the difference between sexes in prevalence of AUD and binge drinking has narrowed. Recent evidence adds to historical data regarding the influence of sex steroids on alcohol drinking and the interaction with stress-related steroids. This review considers the contribution of the endocrine system to alcohol drinking in females, with a focus on the hypothalamic pituitary gonadal axis and the hypothalamic pituitary adrenal axis and their reciprocal interactions. Emphasis is given to preclinical studies that examined genomic and rapid membrane effects of estrogen, progesterone, glucocorticoids, and GABAergic neurosteroids for their effects on alcohol drinking and models of relapse. Pertinent comparisons to data in males highlight divergent effects of sex and stress steroids on alcohol drinking and emphasize the importance of considering sex in the development of novel pharmacotherapeutic targets for the treatment of AUD. For instance, pharmacological strategies targeting the corticotropin releasing factor and glucocorticoid receptor systems may be differentially effective in males and females, whereas strategies to enhance GABAergic neurosteroids may represent a biomarker of treatment efficacy in both sexes.
Keywords: estrogen; ethanol; glucocorticoid; neurosteroid; progesterone; stress.
Conflict of interest statement
Financial disclosure The author declares no competing financial interests.
Figures
References
-
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Use Disorder: A Comparison Between DSM-IV and DSM-5. 2016. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol....
-
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts and Statistics. 2018. [Accessed August 22, 2019]. http://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

