Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis
- PMID: 32714857
- PMCID: PMC7344312
- DOI: 10.3389/fonc.2020.00904
Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis
Abstract
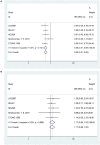
Background: Tyrosine kinase inhibitors (TKIs) are standard treatment options for non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. Increasing clinical investigations have explored the value of EGFR-TKIs plus antiangiogenic drugs as the first-line treatment for EGFR-mutated NSCLC. Methods: We systematically searched PubMed, Cochrane Library, and EMBASE for randomized controlled trials (RCTs) investigating EGFR-TKIs administered with or without antiangiogenic agents for advanced EGFR-mutated NSCLC. The latest RCT that was presented orally at the 2019 European Society for Medical Oncology Congress was obtained online. The endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rates (DCRs), and grade 3 or higher adverse events (AEs). Results: We included seven articles on five trials with 1,226 patients. The interventions for the experimental group were the first-generation EGFR-TKI erlotinib combined with bevacizumab (four studies) or ramucirumab (one study), and erlotinib monotherapy (four studies) or erlotinib plus placebo (one study) for the control group. All studies reached their primary study endpoints (i.e., PFS). Compared to erlotinib monotherapy, erlotinib plus antiangiogenic agents remarkably prolonged PFS [hazard ratio (HR) = 0.59, 95% confidence interval (CI) = 0.51-0.69, P = 0.000]; however, ORR, DCR, and OS were similar between the two groups. The overall grade 3-5 AEs increased in combination group (OR = 5.772, 95% CI = 2.38-13.94, P = 0.000), particularly the incidence of diarrhea (OR = 2.51, 95% CI = 1.21-5.23, P = 0.014), acneiform (OR = 1.815, 95% CI = 1.084-3.037, P = 0.023), hypertension (OR = 6.77, 95% CI = 3.62-12.66, P = 0.000), and proteinuria (OR = 13.48, 95% CI = 4.11-44.22, P = 0.000). Additionally, subgroup analysis demonstrated that Asian patients could significantly benefit from combination therapy (HR = 0.59, 95% CI = 0.50-0.69, P = 0.000). Patients with exon 19 deletions (HR = 0.61, 95% CI = 0.49-0.75, P = 0.000) and 21 Leu858Arg mutations (HR = 0.59, 95% CI = 0.47-0.73, P = 0.000) had almost equivalent PFS benefits when treated with double-blocking therapy. Patients with brain metastases at baseline in the combination group had a trend toward better PFS (HR = 0.55, 95% CI = 0.30-1.01, P = 0.001). Conclusions: Erlotinib plus bevacizumab or ramucirumab in EFGR-mutated NSCLC first-line setting yielded remarkable PFS benefits; however, this was accompanied by higher AEs. Epidermal growth factor receptor-TKI plus antiangiogenic agent therapy may be considered a new option for advanced EGFR-mutated NSCLC patients.
Keywords: EGFR mutation; angiogenesis inhibitors; anti-VEGF; epidermal growth factor receptor inhibitors; first line; meta-analysis; non-small cell lung cancer; targeted treatment.
Copyright © 2020 Chen, Chen, Yu and Cui.
Figures





References
-
- Park K, Tan EH, O'byrne K, Zhang L, Boyer M, Mok T, et al. . Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. (2016) 17:577–89. 10.1016/S1470-2045(16)30033-X - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

