Long-Term Exposure to Ceftriaxone Sodium Induces Alteration of Gut Microbiota Accompanied by Abnormal Behaviors in Mice
- PMID: 32714875
- PMCID: PMC7344183
- DOI: 10.3389/fcimb.2020.00258
Long-Term Exposure to Ceftriaxone Sodium Induces Alteration of Gut Microbiota Accompanied by Abnormal Behaviors in Mice
Abstract
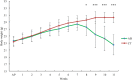
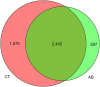
Background: Growing evidence points out that a disturbance of gut microbiota may also disturb the gut-brain communication. However, it is not clear to what extent the alteration of microbiota composition can modulate brain function, affecting host behaviors. Here, we investigated the effects of gut microbiota depletion on emotional behaviors. Methods: Mice in the experimental group were orally administered ceftriaxone sodium solution (250 mg/ml, 0.2 ml/d) for 11 weeks. The open-field test and tail-suspension test were employed for the neurobehavioral assessment of the mice. Fecal samples were collected for 16s rDNA sequencing. The serum levels of cytokines and corticosterone were quantified using enzyme-linked immunosorbent assays. The immunohistochemistry method was used for the detection of brain-derived neurotrophic factor (BDNF) and c-Fos protein. Results: The gut microbiota for antibiotic-treated mice showed lower richness and diversity compared with normal controls. This effect was accompanied by increased anxiety-like, depression-like, and aggressive behaviors. We found these changes to be possibly associated with a dysregulation of the immune system, abnormal activity of the hypothalamic-pituitary-adrenal axis, and an alteration of neurochemistry. Conclusions: The findings demonstrate the indispensable role of microbiota in the gut-brain communication and suggest that the absence of conventional gut microbiota could affect the nervous system, influencing brain function.
Keywords: aggressive behavior; anxiety; ceftriaxone sodium; depression; emotional behaviors; gut microbiota.
Copyright © 2020 Zhao, Wang, Mu, Wang, Luo, Yang, Yang, Li, Zhou and Tao.
Figures










References
-
- Aleshukina A. V. (2012). Pathogenesis of intestinal dysbacteriosis. Zh. Mikrobiol. Epidemiol. Immunobiol. 3, 74–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

