Adaptation and Survival of Burkholderia cepacia and B. contaminans During Long-Term Incubation in Saline Solutions Containing Benzalkonium Chloride
- PMID: 32714902
- PMCID: PMC7344210
- DOI: 10.3389/fbioe.2020.00630
Adaptation and Survival of Burkholderia cepacia and B. contaminans During Long-Term Incubation in Saline Solutions Containing Benzalkonium Chloride
Abstract
The Burkholderia cepacia complex (Bcc) is a group of opportunistic pathogenic bacteria with a remarkable metabolic capacity and broad genotypic/phenotypic plasticity, allowing their adaptation to hostile conditions, including nutrient depleted solutions containing antimicrobial agents. Bcc bacteria are feared contaminants in pharmaceutical industries and cause nosocomial outbreaks, posing health threats to immunocompromised individuals and cystic fibrosis (CF) patients. In this study, the adaptation and survival of B. cepacia and B. contaminans isolates was investigated after long-term incubation in nutrient depleted saline solutions supplemented with increasing concentrations of the biocidal preservative benzalkonium chloride (BZK), recreating the storage conditions of pharmaceutical products. These epidemiologically related isolates were recovered from intrinsically contaminated saline solutions for nasal application and from two CF patients. Long-term incubation in saline solutions containing BZK led to the development of bacterial sub-populations that survived for at least 16 months, despite an initial 2-3 log decrease in viability, displaying a progressive dose-dependent decrease of colony and cell size, including the appearance of small colony variants (SCVs). Bacterial colonies lost pigmentation, changed the morphotype from rough to smooth and produced more spherical cells during extended incubation with BZK. The development of macroscopically visible cellular aggregates, rich in polysaccharide and harboring viable cells in their interior was triggered by BZK. The existence of a metabolic pathway for BZK degradation was confirmed through genome analysis. This study reveals mechanisms underlying the prevalence of Bcc bacteria as contaminants of pharmaceutical products containing BZK, which often lead to false-negative results during quality control and routine testing.
Keywords: Burkholderia cepacia; Burkholderia cepacia complex; Burkholderia contaminans; benzalkonium chloride adaptation; nutrient starvation adaptation; pharmaceutical products' contamination; small colony variants.
Copyright © 2020 Tavares, Kozak, Balola, Coutinho, Godinho, Hassan, Cooper and Sá-Correia.
Figures




 0.05% BZK) and percentage of smaller-sized colonies (
0.05% BZK) and percentage of smaller-sized colonies ( % Smaller-sized colonies 0.05% BZK) obtained for the B. cepacia bacterial populations during 16 months of incubation in saline solutions containing 0.05% BZK. The percentage of smaller-sized colonies only started to be registered at the 82nd day. Cell viability values hereby plotted correspond to those represented in Figure 1.
% Smaller-sized colonies 0.05% BZK) obtained for the B. cepacia bacterial populations during 16 months of incubation in saline solutions containing 0.05% BZK. The percentage of smaller-sized colonies only started to be registered at the 82nd day. Cell viability values hereby plotted correspond to those represented in Figure 1.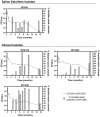
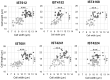
 0.05% BZK) and percentage of smaller-sized colonies (
0.05% BZK) and percentage of smaller-sized colonies ( % Smaller-sized colonies 0.05% BZK) obtained for the B. contaminans bacterial populations during 16 months of incubation in saline solutions containing 0.05% BZK. The percentage of smaller-sized colonies only started to be registered at the 82nd day. Cell viability values hereby plotted correspond to those represented in Figure 2.
% Smaller-sized colonies 0.05% BZK) obtained for the B. contaminans bacterial populations during 16 months of incubation in saline solutions containing 0.05% BZK. The percentage of smaller-sized colonies only started to be registered at the 82nd day. Cell viability values hereby plotted correspond to those represented in Figure 2.
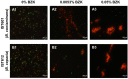
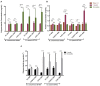

References
-
- Ali M. (2016). Burkholderia cepacia in pharmaceutical industries. Int. J. Vaccines Vaccin. 3:00064 10.15406/ijvv.2016.03.00064 - DOI
-
- Avgeri S. G., Matthaiou D. K., Dimopoulos G., Grammatikos A. P., Falagas M. E. (2009). Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int. J. Antimicrob. Agents 33, 394–404. 10.1016/j.ijantimicag.2008.09.010 - DOI - PubMed
LinkOut - more resources
Full Text Sources

