Application of an Inclined Settler for Cell Culture-Based Influenza A Virus Production in Perfusion Mode
- PMID: 32714908
- PMCID: PMC7343718
- DOI: 10.3389/fbioe.2020.00672
Application of an Inclined Settler for Cell Culture-Based Influenza A Virus Production in Perfusion Mode
Abstract
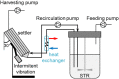
Influenza viruses have been successfully propagated using a variety of animal cell lines in batch, fed-batch, and perfusion culture. For suspension cells, most studies reported on membrane-based cell retention devices typically leading to an accumulation of viruses in the bioreactor in perfusion mode. Aiming at continuous virus harvesting for improved productivities, an inclined settler was evaluated for influenza A virus (IAV) production using the avian suspension cell line AGE1.CR.pIX. Inclined settlers present many advantages as they are scalable, robust, and comply with cGMP regulations, e.g., for recombinant protein manufacturing. Perfusion rates up to 3000 L/day have been reported. In our study, successful growth of AGE1.CR.pIX cells up to 50 × 106 cells/mL and a cell retention efficiency exceeding 96% were obtained with the settler cooled to room temperature. No virus retention was observed. A total of 5.4-6.5 × 1013 virions were produced while a control experiment with an ATF system equaled to 1.9 × 1013 virions. For infection at 25 × 106 cells/mL, cell-specific virus yields up to 3474 virions/cell were obtained, about 5-fold higher than for an ATF based cultivation performed as a control (723 virions/cell). Trypsin activity was shown to have a large impact on cell growth dynamics after infection following the cell retention device, especially at a cell concentration of 50 × 106 cells/mL. Further control experiments performed with an acoustic settler showed that virus production was improved with a heat exchanger of the inclined settler operated at 27°C. In summary, cell culture-based production of viruses in perfusion mode with an inclined settler and continuous harvesting can drastically increase IAV yields and possibly the yield of other viruses. To our knowledge, this is the first report to show the potential of this device for viral vaccine production.
Keywords: continuous harvesting; inclined settler; influenza vaccine; perfusion; suspension cell culture.
Copyright © 2020 Coronel, Gränicher, Sandig, Noll, Genzel and Reichl.
Figures
 ), IS2 (
), IS2 ( ), IS3 (
), IS3 ( ), IS4 (
), IS4 ( ), IS5 (
), IS5 ( ), and IS6 (
), and IS6 ( ). Cultivation with the ATF system (
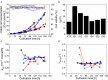
). Cultivation with the ATF system ( ). (A) Viable cell concentration (filled symbols) and cell viability (empty symbols). (B) Doubling time (td) during the cell growth phase. (C) Cell-specific glucose consumption rate (qglc) during perfusion (after 48 h). (D) Lactate yield based on glucose consumption (Ylac/glc) during perfusion (after 48 h).
). (A) Viable cell concentration (filled symbols) and cell viability (empty symbols). (B) Doubling time (td) during the cell growth phase. (C) Cell-specific glucose consumption rate (qglc) during perfusion (after 48 h). (D) Lactate yield based on glucose consumption (Ylac/glc) during perfusion (after 48 h).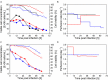
 ), IS4 (
), IS4 ( ), IS5 (
), IS5 ( ), and IS6 (
), and IS6 ( ) plus one control run with an ATF system (
) plus one control run with an ATF system ( ) were carried out. (A, B) Cells were infected at 25 × 106 cells/mL (IS3, IS4, and ATF) or (C, D) 50 × 106 cells/mL (IS5, IS6). (A, C) Viable cell concentration (filled symbols) and cell viability (empty symbols) shown as average of analytical duplicates. (B, D) Perfusion rate in bioreactor working volume per day (day–1).
) were carried out. (A, B) Cells were infected at 25 × 106 cells/mL (IS3, IS4, and ATF) or (C, D) 50 × 106 cells/mL (IS5, IS6). (A, C) Viable cell concentration (filled symbols) and cell viability (empty symbols) shown as average of analytical duplicates. (B, D) Perfusion rate in bioreactor working volume per day (day–1).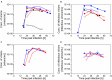
 ), IS4 (
), IS4 ( ), IS5 (
), IS5 ( ), IS6 (
), IS6 ( ), and ATF system (
), and ATF system ( ). (A, C) Concentration of virions in the bioreactor and in the harvest, based on HA titer; (B, D) concentration of infectious virions, based on TCID50. The samples were taken from the bioreactor (filled symbols) and the harvest (empty symbols).
). (A, C) Concentration of virions in the bioreactor and in the harvest, based on HA titer; (B, D) concentration of infectious virions, based on TCID50. The samples were taken from the bioreactor (filled symbols) and the harvest (empty symbols).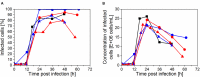
 ), IS4 (
), IS4 ( ), IS5 (
), IS5 ( ), IS6 (
), IS6 ( ), and ATF (
), and ATF ( ).
).
 ) or with heat exchanger (AS2,
) or with heat exchanger (AS2,  ), compared to runs IS3 (
), compared to runs IS3 ( ), IS4 (
), IS4 ( ), and ATF (
), and ATF ( ). The total number of virions produced was normalized to a bioreactor working volume of 650 mL (see section “Yield and titer calculations”).
). The total number of virions produced was normalized to a bioreactor working volume of 650 mL (see section “Yield and titer calculations”).Similar articles
-
Performance of an acoustic settler versus a hollow fiber-based ATF technology for influenza virus production in perfusion.Appl Microbiol Biotechnol. 2020 Jun;104(11):4877-4888. doi: 10.1007/s00253-020-10596-x. Epub 2020 Apr 15. Appl Microbiol Biotechnol. 2020. PMID: 32291490 Free PMC article.
-
High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells.Vaccine. 2014 May 19;32(24):2770-81. doi: 10.1016/j.vaccine.2014.02.016. Epub 2014 Feb 25. Vaccine. 2014. PMID: 24583003
-
Influenza A virus production in a single-use orbital shaken bioreactor with ATF or TFF perfusion systems.Vaccine. 2019 Nov 8;37(47):7011-7018. doi: 10.1016/j.vaccine.2019.06.005. Epub 2019 Jun 29. Vaccine. 2019. PMID: 31266669
-
Potential of cell retention techniques for large-scale high-density perfusion culture of suspended mammalian cells.Biotechnol Bioeng. 2003 Jun 30;82(7):751-65. doi: 10.1002/bit.10629. Biotechnol Bioeng. 2003. PMID: 12701141 Review.
-
Designing cell lines for viral vaccine production: Where do we stand?Biotechnol J. 2015 May;10(5):728-40. doi: 10.1002/biot.201400388. Epub 2015 Apr 22. Biotechnol J. 2015. PMID: 25903999 Review.
Cited by
-
Experimental studies from shake flasks to 3 L stirred tank bioreactor of nutrients and oxygen supply conditions to improve the growth of the avian cell line DuckCelt®-T17.J Biol Eng. 2023 Apr 24;17(1):31. doi: 10.1186/s13036-023-00349-5. J Biol Eng. 2023. PMID: 37095522 Free PMC article.
-
Production of Modified Vaccinia Ankara Virus by Intensified Cell Cultures: A Comparison of Platform Technologies for Viral Vector Production.Biotechnol J. 2021 Jan;16(1):e2000024. doi: 10.1002/biot.202000024. Epub 2020 Sep 8. Biotechnol J. 2021. PMID: 32762152 Free PMC article.
-
Transcriptomic Characterization Reveals Attributes of High Influenza Virus Productivity in MDCK Cells.Viruses. 2021 Nov 1;13(11):2200. doi: 10.3390/v13112200. Viruses. 2021. PMID: 34835006 Free PMC article.
-
Intensified Influenza Virus Production in Suspension HEK293SF Cell Cultures Operated in Fed-Batch or Perfusion with Continuous Harvest.Vaccines (Basel). 2023 Dec 5;11(12):1819. doi: 10.3390/vaccines11121819. Vaccines (Basel). 2023. PMID: 38140223 Free PMC article.
-
From single-cell cloning to high-yield influenza virus production - implementing advanced technologies in vaccine process development.Eng Life Sci. 2024 Feb 18;24(4):2300245. doi: 10.1002/elsc.202300245. eCollection 2024 Apr. Eng Life Sci. 2024. PMID: 38584687 Free PMC article.
References
-
- Berrios J., Altamirano C., Osses N., Gonzalez R. (2011). Continuous CHO cell cultures with improved recombinant protein productivity by using mannose as carbon source: metabolic analysis and scale-up simulation. Chem. Eng. Sci. 66 2431–2439. 10.1016/j.ces.2011.03.011 - DOI
-
- Castilho L. R. (2014). “Continuous animal cell perfusion processes: the first step toward integrated continuous biomanufacturing,” in Continuous Processing in Pharmaceutical Manufacturing, Vol. 1 ed. Subramanian G. (Weinheim: Wiley-VCH; ), 115–154. 10.1002/9783527673681.ch06 - DOI
LinkOut - more resources
Full Text Sources

