Phosphodiesterase Inhibitors for Alzheimer's Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale
- PMID: 32715279
- PMCID: PMC7369141
- DOI: 10.3233/ADR-200191
Phosphodiesterase Inhibitors for Alzheimer's Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale
Abstract
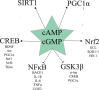
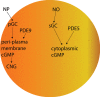
Background: Preclinical studies, clinical trials, and reviews suggest increasing 3',5'-cyclic adenosine monophosphate (cAMP) and 3',5'-cyclic guanosine monophosphate (cGMP) with phosphodiesterase inhibitors is disease-modifying in Alzheimer's disease (AD). cAMP/protein kinase A (PKA) and cGMP/protein kinase G (PKG) signaling are disrupted in AD. cAMP/PKA and cGMP/PKG activate cAMP response element binding protein (CREB). CREB binds mitochondrial and nuclear DNA, inducing synaptogenesis, memory, and neuronal survival gene (e.g., brain-derived neurotrophic factor) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α). cAMP/PKA and cGMP/PKG activate Sirtuin-1, which activates PGC1α. PGC1α induces mitochondrial biogenesis and antioxidant genes (e.g.,Nrf2) and represses BACE1. cAMP and cGMP inhibit BACE1-inducing NFκB and tau-phosphorylating GSK3β.
Objective and methods: We review efficacy-testing clinical trials, epidemiology, and meta-analyses to critically investigate whether phosphodiesteraseinhibitors prevent or treat AD.
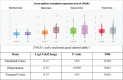
Results: Caffeine and cilostazol may lower AD risk. Denbufylline and sildenafil clinical trials are promising but preliminary and inconclusive. PF-04447943 and BI 409,306 are ineffective. Vinpocetine, cilostazol, and nicergoline trials are mixed. Deprenyl/selegiline trials show only short-term benefits. Broad-spectrum phosphodiesterase inhibitor propentofylline has been shown in five phase III trials to improve cognition, dementia severity, activities of daily living, and global assessment in mild-to-moderate AD patients on multiple scales, including the ADAS-Cogand the CIBIC-Plus in an 18-month phase III clinical trial. However, two books claimed based on a MedScape article an 18-month phase III trial failed, so propentofylline was discontinued. Now, propentofylline is used to treat canine cognitive dysfunction, which, like AD, involves age-associated wild-type Aβ deposition.
Conclusion: Phosphodiesterase inhibitors may prevent and treat AD.
Keywords: 3′, 5′-cyclic-AMP phosphodiesterases; 3′, 5′-cyclic-GMP phosphodiesterases; Alzheimer’s disease; NF-E2-related factor 2; NF-kappa B; Sirtuin 1; amyloid precursor protein secretases; clinical trial phase III; cyclic AMP response element-binding protein; cyclic nucleotides; glycogen synthase kinase 3; peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
© 2020 – IOS Press and the authors. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures







References
-
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. - PubMed
-
- Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WST, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA, Del K, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA (2017) Microbes and Alzheimer’s disease. J Alzheimers Dis 51, 979–984. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

