The use of 5-aminosalicylate for patients with Crohn's disease in a prospective European inception cohort with 5 years follow-up - an Epi-IBD study
- PMID: 32715989
- PMCID: PMC7707880
- DOI: 10.1177/2050640620945949
The use of 5-aminosalicylate for patients with Crohn's disease in a prospective European inception cohort with 5 years follow-up - an Epi-IBD study
Abstract
Background: The lack of scientific evidence regarding the effectiveness of 5-aminosalicylate in patients with Crohn's disease is in sharp contrast to its widespread use in clinical practice.
Aims: The aim of the study was to investigate the use of 5-aminosalicylate in patients with Crohn's disease as well as the disease course of a subgroup of patients who were treated with 5-aminosalicylate as maintenance monotherapy during the first year of disease.
Methods: In a European community-based inception cohort, 488 patients with Crohn's disease were followed from the time of their diagnosis. Information on clinical data, demographics, disease activity, medical therapy and rates of surgery, cancers and deaths was collected prospectively. Patient management was left to the discretion of the treating gastroenterologists.
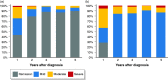
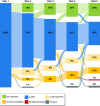
Results: Overall, 292 (60%) patients with Crohn's disease received 5-aminosalicylate period during follow-up for a median duration of 28 months (interquartile range 6-60). Of these, 78 (16%) patients received 5-aminosalicylate monotherapy during the first year following diagnosis. Patients who received monotherapy with 5-aminosalicylate experienced a mild disease course with only nine (12%) who required hospitalization, surgery, or developed stricturing or penetrating disease, and most never needed more intensive therapy. The remaining 214 patients were treated with 5-aminosalicylate as the first maintenance drug although most eventually needed to step up to other treatments including immunomodulators (75 (35%)), biological therapy (49 (23%)) or surgery (38 (18%)).
Conclusion: In this European community-based inception cohort of unselected Crohn's disease patients, 5-aminosalicylate was commonly used. A substantial group of these patients experienced a quiescent disease course without need of additional treatment during follow-up. Therefore, despite the controversy regarding the efficacy of 5-aminosalicylate in Crohn's disease, its use seems to result in a satisfying disease course for both patients and physicians.
Keywords: 5-aminosalicylates; Population-based cohort; disease course.
Figures

References
-
- Munkholm P, Langholz E, Davidsen M, et al. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 1995; 30: 699–706. - PubMed
-
- Ford AC, Kane S V, Khan KJ, et al. Efficacy of 5-aminosalicylates in Crohn’s disease: Systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 617–629. - PubMed
-
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J Crohns Colitis 2020; 14: 4--22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

