Continuous vs Routine Electroencephalogram in Critically Ill Adults With Altered Consciousness and No Recent Seizure: A Multicenter Randomized Clinical Trial
- PMID: 32716479
- PMCID: PMC7385681
- DOI: 10.1001/jamaneurol.2020.2264
Continuous vs Routine Electroencephalogram in Critically Ill Adults With Altered Consciousness and No Recent Seizure: A Multicenter Randomized Clinical Trial
Abstract
Importance: In critically ill patients with altered consciousness, continuous electroencephalogram (cEEG) improves seizure detection, but is resource-consuming compared with routine EEG (rEEG). It is also uncertain whether cEEG has an effect on outcome.
Objective: To assess whether cEEG is associated with reduced mortality compared with rEEG.
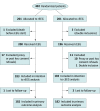
Design, setting, and participants: The pragmatic multicenter Continuous EEG Randomized Trial in Adults (CERTA) was conducted between 2017 and 2018, with follow-up of 6 months. Outcomes were assessed by interviewers blinded to interventions.The study took place at 4 tertiary hospitals in Switzerland (intensive and intermediate care units). Depending on investigators' availability, we pragmatically recruited critically ill adults having Glasgow Coma Scale scores of 11 or less or Full Outline of Responsiveness score of 12 or less, without recent seizures or status epilepticus. They had cerebral (eg, brain trauma, cardiac arrest, hemorrhage, or stroke) or noncerebral conditions (eg, toxic-metabolic or unknown etiology), and EEG was requested as part of standard care. An independent physician provided emergency informed consent.
Interventions: Participants were randomized 1:1 to cEEG for 30 to 48 hours vs 2 rEEGs (20 minutes each), interpreted according to standardized American Clinical Neurophysiology Society guidelines.
Main outcomes and measures: Mortality at 6 months represented the primary outcome. Secondary outcomes included interictal and ictal features detection and change in therapy.
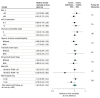
Results: We analyzed 364 patients (33% women; mean [SD] age, 63 [15] years). At 6 months, mortality was 89 of 182 in those with cEEG and 88 of 182 in those with rEEG (adjusted relative risk [RR], 1.02; 95% CI, 0.83-1.26; P = .85). Exploratory comparisons within subgroups stratifying patients according to age, premorbid disability, comorbidities on admission, deeper consciousness reduction, and underlying diagnoses revealed no significant effect modification. Continuous EEG was associated with increased detection of interictal features and seizures (adjusted RR, 1.26; 95% CI, 1.08-1.15; P = .004 and 3.37; 95% CI, 1.63-7.00; P = .001, respectively) and more frequent adaptations in antiseizure therapy (RR, 1.84; 95% CI, 1.12-3.00; P = .01).
Conclusions and relevance: This pragmatic trial shows that in critically ill adults with impaired consciousness and no recent seizure, cEEG leads to increased seizure detection and modification of antiseizure treatment but is not related to improved outcome compared with repeated rEEG. Pending larger studies, rEEG may represent a valid alternative to cEEG in centers with limited resources.
Trial registration: ClinicalTrials.gov Identifier: NCT03129438.
Conflict of interest statement
Figures
Comment in
-
Continuous Electroencephalogram-Necessity or Luxury?JAMA Neurol. 2020 Oct 1;77(10):1211-1212. doi: 10.1001/jamaneurol.2020.1483. JAMA Neurol. 2020. PMID: 32716477 No abstract available.
-
Assessment of a Study of Continuous vs Repeat-Spot Electroencephalography in Patients With Critical Illness-Reply.JAMA Neurol. 2021 Mar 1;78(3):369-370. doi: 10.1001/jamaneurol.2020.5343. JAMA Neurol. 2021. PMID: 33523097 No abstract available.
-
Assessment of a Study of Continuous vs Repeat-Spot Electroencephalography in Patients With Critical Illness.JAMA Neurol. 2021 Mar 1;78(3):369. doi: 10.1001/jamaneurol.2020.5348. JAMA Neurol. 2021. PMID: 33523103 Free PMC article. No abstract available.
-
Continuous EEG in ICU: Not a Luxury After All.Epilepsy Curr. 2020 Nov 23;21(1):21-23. doi: 10.1177/1535759720973987. eCollection 2021 Jan-Feb. Epilepsy Curr. 2020. PMID: 34025267 Free PMC article. No abstract available.
References
-
- Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, Oddo M; Neurointensive Care Section of the European Society of Intensive Care Medicine . Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39(8):1337-1351. doi:10.1007/s00134-013-2938-4 - DOI - PubMed
-
- Herman ST, Abend NS, Bleck TP, et al. ; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society . Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32(2):96-108. doi:10.1097/WNP.0000000000000165 - DOI - PMC - PubMed
-
- Herman ST, Abend NS, Bleck TP, et al. ; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society . Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95. doi:10.1097/WNP.0000000000000166 - DOI - PMC - PubMed

