Safety, Tolerability, and Immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (PCV20) in Adults 60 to 64 Years of Age
- PMID: 32716500
- PMCID: PMC8492133
- DOI: 10.1093/cid/ciaa1045
Safety, Tolerability, and Immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (PCV20) in Adults 60 to 64 Years of Age
Abstract
Background: Pneumococcal conjugate vaccines (PCVs) have significantly decreased pneumococcal disease worldwide; however, expanding serotype coverage may further reduce disease burden. A 20-valent PCV (PCV20) containing capsular polysaccharide conjugates of serotypes present in the 13-valent PCV (PCV13) and 7 new serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) is currently in development. This phase 2 study evaluated safety, tolerability, and immunogenicity of PCV20 in adults without prior pneumococcal vaccination.
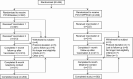
Methods: In this randomized, active-controlled, double-blinded trial, 444 adults 60 through 64 years of age were randomized to receive either a single dose of PCV20 followed 1 month later by saline placebo or a single dose of PCV13 followed 1 month later by 23-valent polysaccharide vaccine. Local injection site reactions, select systemic symptoms, and adverse events (AEs) were recorded. Immunogenicity was assessed by measuring serotype-specific opsonophagocytic activity (OPA) titers before and approximately 1 month after each vaccination.
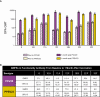
Results: Local reaction and systemic event rates were similar after vaccination with PCV20 or PCV13; no serious vaccine-related AEs were reported. In the PCV20 group, functional immune responses as measured by OPA were robust for all 20 serotypes included in the vaccine, with geometric mean fold rises from baseline ranging from 6.0 to 113.4.
Conclusions: PCV20 was well tolerated in adults 60 to 64 years of age, with a safety profile consistent with historical experience of PCVs in this age group. Substantial OPA responses were elicited against all serotypes. Results demonstrate the potential for PCV20 to expand pneumococcal disease protection.
Clinical trials registration: NCT03313037.
Keywords: Streptococcus pneumoniae; 20-valent pneumococcal conjugate vaccine; adult; clinical trial; immunogenicity and safety.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- World Health Organization. Pneumococcal vaccines WHO position paper 2012. Wkly Epidemiol Rec 2012; 87:129–44. - PubMed
-
- Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect 2012; 18:7–14. - PubMed
-
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20:45–51. - PubMed
-
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis 2003; 187:1424–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

