Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma
- PMID: 32716739
- PMCID: PMC7479760
- DOI: 10.1200/JCO.20.00808
Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma
Abstract
Purpose: The immunomodulatory effect of lenvatinib (a multikinase inhibitor) on tumor microenvironments may contribute to antitumor activity when combined with programmed death receptor-1 (PD-1) signaling inhibitors in hepatocellular carcinoma (HCC). We report results from a phase Ib study of lenvatinib plus pembrolizumab (an anti-PD-1 antibody) in unresectable HCC (uHCC).
Patients and methods: In this open-label multicenter study, patients with uHCC received lenvatinib (bodyweight ≥ 60 kg, 12 mg; < 60 kg, 8 mg) orally daily and pembrolizumab 200 mg intravenously on day 1 of a 21-day cycle. The study included a dose-limiting toxicity (DLT) phase and an expansion phase (first-line patients). Primary objectives were safety/tolerability (DLT phase), and objective response rate (ORR) and duration of response (DOR) by modified RECIST (mRECIST) and RECIST version 1.1 (v1.1) per independent imaging review (IIR; expansion phase).
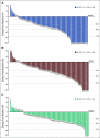
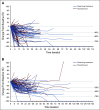
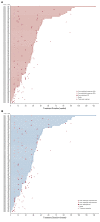
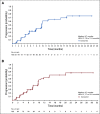
Results: A total of 104 patients were enrolled. No DLTs were reported (n = 6) in the DLT phase; 100 patients (expansion phase; included n = 2 from DLT phase) had received no prior systemic therapy and had Barcelona Clinic Liver Cancer stage B (n = 29) or C disease (n = 71). At data cutoff, 37% of patients remained on treatment. Median duration of follow-up was 10.6 months (95% CI, 9.2 to 11.5 months). Confirmed ORRs by IIR were 46.0% (95% CI, 36.0% to 56.3%) per mRECIST and 36.0% (95% CI, 26.6% to 46.2%) per RECIST v1.1. Median DORs by IIR were 8.6 months (95% CI, 6.9 months to not estimable [NE]) per mRECIST and 12.6 months (95% CI, 6.9 months to NE) per RECIST v1.1. Median progression-free survival by IIR was 9.3 months per mRECIST and 8.6 months per RECIST v1.1. Median overall survival was 22 months. Grade ≥ 3 treatment-related adverse events occurred in 67% (grade 5, 3%) of patients. No new safety signals were identified.
Conclusion: Lenvatinib plus pembrolizumab has promising antitumor activity in uHCC. Toxicities were manageable, with no unexpected safety signals.
Trial registration: ClinicalTrials.gov NCT03006926.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. - PubMed
-
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

