Respiratory Tract Dysbiosis Is Associated with Worse Outcomes in Mechanically Ventilated Patients
- PMID: 32717152
- PMCID: PMC7737572
- DOI: 10.1164/rccm.201912-2441OC
Respiratory Tract Dysbiosis Is Associated with Worse Outcomes in Mechanically Ventilated Patients
Abstract
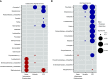
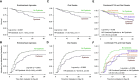
Rationale: Host inflammatory responses have been strongly associated with adverse outcomes in critically ill patients, but the biologic underpinnings of such heterogeneous responses have not been defined.Objectives: We examined whether respiratory tract microbiome profiles are associated with host inflammation and clinical outcomes of acute respiratory failure.Methods: We collected oral swabs, endotracheal aspirates (ETAs), and plasma samples from mechanically ventilated patients. We performed 16S ribosomal RNA gene sequencing to characterize upper and lower respiratory tract microbiota and classified patients into host-response subphenotypes on the basis of clinical variables and plasma biomarkers of innate immunity and inflammation. We derived diversity metrics and composition clusters with Dirichlet multinomial models and examined our data for associations with subphenotypes and clinical outcomes.Measurements and Main Results: Oral and ETA microbial communities from 301 mechanically ventilated subjects had substantial heterogeneity in α and β diversity. Dirichlet multinomial models revealed a cluster with low α diversity and enrichment for pathogens (e.g., high Staphylococcus or Pseudomonadaceae relative abundance) in 35% of ETA samples, associated with a hyperinflammatory subphenotype, worse 30-day survival, and longer time to liberation from mechanical ventilation (adjusted P < 0.05), compared with patients with higher α diversity and relative abundance of typical oral microbiota. Patients with evidence of dysbiosis (low α diversity and low relative abundance of "protective" oral-origin commensal bacteria) in both oral and ETA samples (17%, combined dysbiosis) had significantly worse 30-day survival and longer time to liberation from mechanical ventilation than patients without dysbiosis (55%; adjusted P < 0.05).Conclusions: Respiratory tract dysbiosis may represent an important, modifiable contributor to patient-level heterogeneity in systemic inflammatory responses and clinical outcomes.
Keywords: acute respiratory distress syndrome; bacterial infections; endotypes; inflammation; microbiota.
Figures



Comment in
-
In Pursuit of Microbiome-based Therapies for Acute Respiratory Failure.Am J Respir Crit Care Med. 2020 Dec 15;202(12):1616-1618. doi: 10.1164/rccm.202008-3146ED. Am J Respir Crit Care Med. 2020. PMID: 32910676 Free PMC article. No abstract available.
References
-
- Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5:524–534. - PubMed
Publication types
MeSH terms
Grants and funding
- K24 HL123342/HL/NHLBI NIH HHS/United States
- R01 LM012087/LM/NLM NIH HHS/United States
- R01 HL097376/HL/NHLBI NIH HHS/United States
- U01 HL137159/HL/NHLBI NIH HHS/United States
- U01 HL098962/HL/NHLBI NIH HHS/United States
- K23 GM122069/GM/NIGMS NIH HHS/United States
- F32 HL137258/HL/NHLBI NIH HHS/United States
- R01 HL127349/HL/NHLBI NIH HHS/United States
- IK2 BX004886/BX/BLRD VA/United States
- L30 HL143734/HL/NHLBI NIH HHS/United States
- K23 HL139987/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- F32 HL142172/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

