Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study
- PMID: 32717210
- PMCID: PMC7380925
- DOI: 10.1016/S2213-2600(20)30315-5
Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study
Erratum in
-
Correction to Lancet Respir Med 2020; published online July 24. https://doi.org/10.1016/S2213-2600(20)30315-5.Lancet Respir Med. 2020 Sep;8(9):e71. doi: 10.1016/S2213-2600(20)30350-7. Epub 2020 Jul 30. Lancet Respir Med. 2020. PMID: 32738927 Free PMC article. No abstract available.
Abstract
Background: Health-care workers constitute a high-risk population for acquisition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Capacity for acute diagnosis via PCR testing was limited for individuals with mild to moderate SARS-CoV-2 infection in the early phase of the COVID-19 pandemic and a substantial proportion of health-care workers with suspected infection were not tested. We aimed to investigate the performance of point-of-care and laboratory serology assays and their utility in late case identification, and to estimate SARS-CoV-2 seroprevalence.
Methods: We did a prospective multicentre cohort study between April 8 and June 12, 2020, in two phases. Symptomatic health-care workers with mild to moderate symptoms were eligible to participate 14 days after onset of COVID-19 symptoms, as per the Public Health England (PHE) case definition. Health-care workers were recruited to the asymptomatic cohort if they had not developed PHE-defined COVID-19 symptoms since Dec 1, 2019. In phase 1, two point-of-care lateral flow serological assays, the Onsite CTK Biotech COVID-19 split IgG/IgM Rapid Test (CTK Bitotech, Poway, CA, USA) and the Encode SARS-CoV-2 split IgM/IgG One Step Rapid Test Device (Zhuhai Encode Medical Engineering, Zhuhai, China), were evaluated for performance against a laboratory immunoassay (EDI Novel Coronavirus COVID-19 IgG ELISA kit [Epitope Diagnostics, San Diego, CA, USA]) in 300 samples from health-care workers and 100 pre-COVID-19 negative control samples. In phase 2 (n=6440), serosurveillance was done among 1299 (93·4%) of 1391 health-care workers reporting symptoms, and in a subset of asymptomatic health-care workers (405 [8·0%] of 5049).
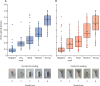
Findings: There was variation in test performance between the lateral flow serological assays; however, the Encode assay displayed reasonable IgG sensitivity (127 of 136; 93·4% [95% CI 87·8-96·9]) and specificity (99 of 100; 99·0% [94·6-100·0]) among PCR-proven cases and good agreement (282 of 300; 94·0% [91·3-96·7]) with the laboratory immunoassay. By contrast, the Onsite assay had reduced sensitivity (120 of 136; 88·2% [95% CI 81·6-93·1]) and specificity (94 of 100; 94·0% [87·4-97·8]) and agreement (254 of 300; 84·7% [80·6-88·7]). Five (7%) of 70 PCR-positive cases were negative across all assays. Late changes in lateral flow serological assay bands were recorded in 74 (9·3%) of 800 cassettes (35 [8·8%] of 400 Encode assays; 39 [9·8%] of 400 Onsite assays), but only seven (all Onsite assays) of these changes were concordant with the laboratory immunoassay. In phase 2, seroprevalence among the workforce was estimated to be 10·6% (95% CI 7·6-13·6) in asymptomatic health-care workers and 44·7% (42·0-47·4) in symptomatic health-care workers. Seroprevalence across the entire workforce was estimated at 18·0% (95% CI 17·0-18·9).
Interpretation: Although a good positive predictive value was observed with both lateral flow serological assays and ELISA, this agreement only occurred if the pre-test probability was modified by a strict clinical case definition. Late development of lateral flow serological assay bands would preclude postal strategies and potentially home testing. Identification of false-negative results among health-care workers across all assays suggest caution in interpretation of IgG results at this stage; for now, testing is perhaps best delivered in a clinical setting, supported by government advice about physical distancing.
Funding: None.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- WHO Coronavirus disease (COVID-19). Situation report – 180. July 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Public Health England COVID-19: infection prevention and control guidance. April 24, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

