Effect of Aging on Daily Rhythms of Lactate Metabolism in the Medial Prefrontal Cortex of Male Mice
- PMID: 32717298
- PMCID: PMC7584730
- DOI: 10.1016/j.neuroscience.2020.07.032
Effect of Aging on Daily Rhythms of Lactate Metabolism in the Medial Prefrontal Cortex of Male Mice
Abstract
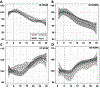
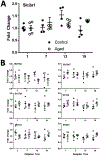
Aging is associated with reduced amplitude and earlier timing of circadian (daily) rhythms in sleep, brain function, and behavior. We examined whether age-related circadian dysfunction extends to the metabolic function of the brain, particularly in the prefrontal cortex (PFC). Using enzymatic amperometric biosensors, we recorded lactate concentration changes in the PFC in Young (7 mos) and Aged (19 mos) freely-behaving C57BL/6N male mice. Both Young and Aged mice displayed diurnal and circadian rhythms of lactate, with the Aged rhythm slightly phase advanced. Under constant conditions, the Aged rhythm showed a reduced amplitude not seen in the Young mice. We simultaneously observed a relationship between arousal state and PFC lactate rhythm via electroencephalography, which was modified by aging. Finally, using RT-qPCR, we found that aging affects the daily expression pattern of Glucose Transporter 1 (GLUT-1).
Keywords: biosensor; circadian; neurometabolism; sleep; solute transporters.
Copyright © 2020 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Interests Statement
The authors report no competing interests.
Figures



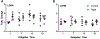
Comment in
-
Rhythms in Neurometabolism Decline with Age.Neuroscience. 2020 Nov 10;448:299. doi: 10.1016/j.neuroscience.2020.09.014. Epub 2020 Sep 14. Neuroscience. 2020. PMID: 32941936 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

