Immunological co-ordination between gut and lungs in SARS-CoV-2 infection
- PMID: 32717345
- PMCID: PMC7380259
- DOI: 10.1016/j.virusres.2020.198103
Immunological co-ordination between gut and lungs in SARS-CoV-2 infection
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved into a major pandemic called coronavirus disease 2019 (COVID-19) that has created unprecedented global health emergencies, and emerged as a serious threat due to its strong ability for human-to-human transmission. The reports indicate the ability of SARS-CoV-2 to affect almost any organ due to the presence of a receptor known as angiotensin converting enzyme 2 (ACE2) across the body. ACE2 receptor is majorly expressed in the brush border of gut enterocytes along with the ciliated cells and alveolar epithelial type II cells in the lungs. The amino acid transport function of ACE2 has been linked to gut microbial ecology in gastrointestinal (GI) tract, thereby suggesting that COVID-19 may, to some level, be linked to the enteric microbiota. The significant number of COVID-19 patients shows extra-pulmonary symptoms in the GI tract. Many subsequent studies revealed viral RNA of SARS-CoV-2 in fecal samples of COVID-19 patients. This presents a new challenge in the diagnosis and control of COVID-19 infection with a caution for proper sanitation and hygiene. Here, we aim to discuss the immunological co-ordination between gut and lungs that facilitates SARS-CoV-2 to infect and multiply in the inflammatory bowel disease (IBD) and non-IBD patients.
Keywords: ACE2; COVID-19; Gastrointestinal tract; Gut–lung axis; Inflammatory bowel disease; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures
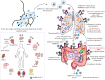
 : ACE2 receptor,
: ACE2 receptor,  : TMPRSS2 receptor,
: TMPRSS2 receptor,  : neutrophils,
: neutrophils,  : lymphocytes,
: lymphocytes,  : mucus, and
: mucus, and  : inflammatory mediators. *ACE2 and TMPRSS2 are expressed in the brush border of host cells. In figure, the localisation of ACE2 and TMPRSS2 outside of the gut or lungs instead of in brush border contact is just for easy representation to the readers.
: inflammatory mediators. *ACE2 and TMPRSS2 are expressed in the brush border of host cells. In figure, the localisation of ACE2 and TMPRSS2 outside of the gut or lungs instead of in brush border contact is just for easy representation to the readers.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

