Reducing monocarboxylate transporter MCT1 worsens experimental diabetic peripheral neuropathy
- PMID: 32717355
- PMCID: PMC7502508
- DOI: 10.1016/j.expneurol.2020.113415
Reducing monocarboxylate transporter MCT1 worsens experimental diabetic peripheral neuropathy
Abstract
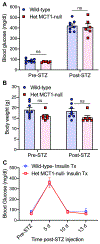
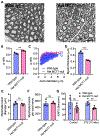
Diabetic peripheral neuropathy (DPN) is one of the most common complications in diabetic patients. Though the exact mechanism for DPN is unknown, it clearly involves metabolic dysfunction and energy failure in multiple cells within the peripheral nervous system. Lactate is an alternate source of metabolic energy that is increasingly recognized for its role in supporting neurons. The primary transporter for lactate in the nervous system, monocarboxylate transporter-1 (MCT1), has been shown to be critical for peripheral nerve regeneration and metabolic support to neurons/axons. In this study, MCT1 was reduced in both sciatic nerve and dorsal root ganglia in wild-type mice treated with streptozotocin (STZ), a common model of type-1 diabetes. Heterozygous MCT1 null mice that developed hyperglycemia following STZ treatment developed a more severe DPN compared to wild-type mice, as measured by greater axonal demyelination, decreased peripheral nerve function, and increased numbness to innocuous low-threshold mechanical stimulation. Given that MCT1 inhibitors are being developed as both immunosuppressive and chemotherapeutic medications, our results suggest that clinical development in patients with diabetes should proceed with caution. Collectively, our findings uncover an important role for MCT1 in DPN and provide a potential lead toward developing novel treatments for this currently untreatable disease.
Keywords: Diabetic peripheral neuropathy; Dorsal root ganglion; Metabolism; Monocarboxylate transporter; Peripheral nerve.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing financial interests.
Figures



Similar articles
-
Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush.Exp Neurol. 2015 Jan;263:325-38. doi: 10.1016/j.expneurol.2014.10.018. Epub 2014 Oct 29. Exp Neurol. 2015. PMID: 25447940 Free PMC article.
-
Pyruvate Dehydrogenase Kinase-mediated Glycolytic Metabolic Shift in the Dorsal Root Ganglion Drives Painful Diabetic Neuropathy.J Biol Chem. 2016 Mar 11;291(11):6011-6025. doi: 10.1074/jbc.M115.699215. Epub 2016 Jan 14. J Biol Chem. 2016. PMID: 26769971 Free PMC article.
-
Na+ /K+ -ATPase coupled to endothelin receptor type B stimulates peripheral nerve regeneration via lactate signalling.Eur J Neurosci. 2017 Sep;46(5):2096-2107. doi: 10.1111/ejn.13647. Epub 2017 Aug 17. Eur J Neurosci. 2017. PMID: 28700113
-
Oligodendroglia: metabolic supporters of axons.Trends Cell Biol. 2013 Dec;23(12):644-51. doi: 10.1016/j.tcb.2013.07.007. Epub 2013 Aug 27. Trends Cell Biol. 2013. PMID: 23988427 Free PMC article. Review.
-
Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes.Nitric Oxide. 2017 Jul 1;67:75-80. doi: 10.1016/j.niox.2017.04.004. Epub 2017 Apr 7. Nitric Oxide. 2017. PMID: 28392448 Review.
Cited by
-
Monocarboxylate Transporters: Role and Regulation in Corneal Diabetes.Anal Cell Pathol (Amst). 2022 Oct 26;2022:6718566. doi: 10.1155/2022/6718566. eCollection 2022. Anal Cell Pathol (Amst). 2022. PMID: 36340268 Free PMC article.
-
Single-cell RNA-seq uncovers novel metabolic functions of Schwann cells beyond myelination.J Neurochem. 2023 Jul;166(2):367-388. doi: 10.1111/jnc.15877. Epub 2023 Jun 16. J Neurochem. 2023. PMID: 37328915 Free PMC article.
-
[Research advances on the role of Schwann cells in diabetic peripheral neuropathy].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023 Dec 20;39(12):1190-1194. doi: 10.3760/cma.j.cn501225-20230727-00019. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023. PMID: 38129308 Free PMC article. Review. Chinese.
-
New perspectives in diabetic neuropathy.Neuron. 2023 Sep 6;111(17):2623-2641. doi: 10.1016/j.neuron.2023.05.003. Epub 2023 May 31. Neuron. 2023. PMID: 37263266 Free PMC article. Review.
-
Glial-neuron crosstalk in health and disease: A focus on metabolism, obesity, and cognitive impairment.Neurobiol Dis. 2022 Aug;170:105766. doi: 10.1016/j.nbd.2022.105766. Epub 2022 May 16. Neurobiol Dis. 2022. PMID: 35584728 Free PMC article. Review.
References
-
- Aghanoori MR, Smith DR, Shariati-Ievari S, Ajisebutu A, Nguyen A, Desmond F, Jesus CHA, Zhou X, Calcutt NA, Aliani M, Fernyhough P, 2019. Insulin-like growth factor-1 activates AMPK to augment mitochondrial function and correct neuronal metabolism in sensory neurons in type 1 diabetes. Mol Metab 20, 149–165. - PMC - PubMed
-
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V, 2019. Diabetic neuropathy. Nat Rev Dis Primers 5, 41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

