Plasmacytoid dendritic cells in the eye
- PMID: 32717378
- PMCID: PMC7854822
- DOI: 10.1016/j.preteyeres.2020.100877
Plasmacytoid dendritic cells in the eye
Abstract
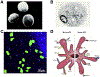
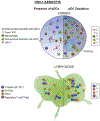
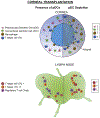
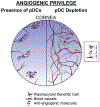
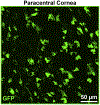
Plasmacytoid dendritic cells (pDCs) are a unique subpopulation of immune cells, distinct from classical dendritic cells. pDCs are generated in the bone marrow and following development, they typically home to secondary lymphoid tissues. While peripheral tissues are generally devoid of pDCs during steady state, few tissues, including the lung, kidney, vagina, and in particular ocular tissues harbor resident pDCs. pDCs were originally appreciated for their potential to produce large quantities of type I interferons in viral immunity. Subsequent studies have now unraveled their pivotal role in mediating immune responses, in particular in the induction of tolerance. In this review, we summarize our current knowledge on pDCs in ocular tissues in both mice and humans, in particular in the cornea, limbus, conjunctiva, choroid, retina, and lacrimal gland. Further, we will review our current understanding on the significance of pDCs in ameliorating inflammatory responses during herpes simplex virus keratitis, sterile inflammation, and corneal transplantation. Moreover, we describe their novel and pivotal neuroprotective role, their key function in preserving corneal angiogenic privilege, as well as their potential application as a cell-based therapy for ocular diseases.
Keywords: Angiogenesis; Neuroprotection; Plasmacytoid dendritic cells; Tolerance; Transplantation; Viral keratitis.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

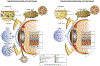




References
-
- Abe M, et al. (2005). “Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival.” Am J Transplant 5(8): 1808–1819. - PubMed
-
- Abou-Slaybi A, et al. (2019). “Analysis of leukocyte populations and nerves in developing murine corneas.” The Journal of Immunology 202(1 Supplement): 117.115–117.115.
-
- Agrawal T, et al. (2009). “Recruitment of myeloid and plasmacytoid dendritic cells in cervical mucosa during Chlamydia trachomatis infection.” Clin Microbiol Infect 15(1): 50–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

