FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy
- PMID: 32717568
- PMCID: PMC7362818
- DOI: 10.1016/j.drup.2020.100719
FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy
Abstract
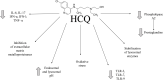
In December 2019, a novel SARS-CoV-2 coronavirus emerged, causing an outbreak of life-threatening pneumonia in the Hubei province, China, and has now spread worldwide, causing a pandemic. The urgent need to control the disease, combined with the lack of specific and effective treatment modalities, call for the use of FDA-approved agents that have shown efficacy against similar pathogens. Chloroquine, remdesivir, lopinavir/ritonavir or ribavirin have all been successful in inhibiting SARS-CoV-2 in vitro. The initial results of a number of clinical trials involving various protocols of administration of chloroquine or hydroxychloroquine mostly point towards their beneficial effect. However, they may not be effective in cases with persistently high viremia, while results on ivermectin (another antiparasitic agent) are not yet available. Interestingly, azithromycin, a macrolide antibiotic in combination with hydroxychloroquine, might yield clinical benefit as an adjunctive. The results of clinical trials point to the potential clinical efficacy of antivirals, especially remdesivir (GS-5734), lopinavir/ritonavir, and favipiravir. Other therapeutic options that are being explored involve meplazumab, tocilizumab, and interferon type 1. We discuss a number of other drugs that are currently in clinical trials, whose results are not yet available, and in various instances we enrich such efficacy analysis by invoking historic data on the treatment of SARS, MERS, influenza, or in vitro studies. Meanwhile, scientists worldwide are seeking to discover novel drugs that take advantage of the molecular structure of the virus, its intracellular life cycle that probably elucidates unfolded-protein response, as well as its mechanism of surface binding and cell invasion, like angiotensin converting enzymes-, HR1, and metalloproteinase inhibitors.
Keywords: Chloroquine; Coronavirus; Lopinavir; Remdesivir; Ribavirin; Ritonavir; SARS-CoV-2.
Crown Copyright © 2020. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Alavian S.M., et al. Virus-triggered autophagy in viral hepatitis - possible novel strategies for drug development. J. Viral Hepat. 2011;18(12):821–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

