CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning
- PMID: 32717742
- PMCID: PMC7554077
- DOI: 10.1038/s41586-020-2578-0
CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning
Abstract
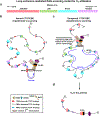
The RAG endonuclease initiates Igh locus V(D)J recombination in progenitor (pro)-B cells1. Upon binding a recombination centre-based JH, RAG scans upstream chromatin via loop extrusion, potentially mediated by cohesin, to locate Ds and assemble a DJH-based recombination centre2. CTCF looping factor-bound elements (CBEs) within IGCR1 upstream of Ds impede RAG scanning3-5; however, their inactivation allows scanning to proximal VHs, where additional CBEs activate rearrangement and impede scanning any further upstream5. Distal VH utilization is thought to involve diffusional access to the recombination centre following large-scale Igh locus contraction6-8. Here we test the potential of linear RAG scanning to mediate distal VH usage in G1-arrested v-Abl pro-B cell lines9, which undergo robust D-to-JH but little VH-to-DJH rearrangements, presumably owing to lack of locus contraction2,5. Through an auxin-inducible approach10, we degraded the cohesin component RAD2110-12 or CTCF12,13 in these G1-arrested lines. Degradation of RAD21 eliminated all V(D)J recombination and interactions associated with RAG scanning, except for reecombination centre-located DQ52-to-JH joining, in which synapsis occurs by diffusion2. Remarkably, while degradation of CTCF suppressed most CBE-based chromatin interactions, it promoted robust recombination centre interactions with, and robust VH-to-DJH joining of, distal VHs, with patterns similar to those of 'locus-contracted' primary pro-B cells. Thus, downmodulation of CTCF-bound scanning-impediment activity promotes cohesin-driven RAG scanning across the 2.7-Mb Igh locus.
Figures










References
-
- Teng G & Schatz DG Regulation and Evolution of the RAG Recombinase. Adv. Immunol. 128, 1–39 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

