Antenatal Corticosteroids and Preterm Neonatal Morbidity and Mortality among Women with and without Diabetes in Pregnancy
- PMID: 32717749
- PMCID: PMC7854806
- DOI: 10.1055/s-0040-1714391
Antenatal Corticosteroids and Preterm Neonatal Morbidity and Mortality among Women with and without Diabetes in Pregnancy
Abstract
Objective: The objective of this study was to determine whether antenatal corticosteroid exposure has a differential association with preterm neonatal morbidity among women with and without diabetes.
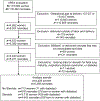
Study design: Secondary analysis of an observational cohort of 115,502 women and their neonates born in 25 U.S. hospitals (2008-2011). Women who delivered at 230/7 to 336/7 weeks' gestation and received antenatal corticosteroids were compared with those who did not receive antenatal corticosteroids. Women with a stillbirth and women who delivered a neonate that was not resuscitated were excluded. The primary outcome was neonatal respiratory distress syndrome or death within 48 hours. Secondary outcomes included composite neonatal morbidity (respiratory distress syndrome, necrotizing enterocolitis, grades 3-4 intraventricular hemorrhage, sepsis, or death) and mechanical ventilation. Multivariable modified Poisson regression was used to estimate the association between antenatal corticosteroid exposure and neonatal outcomes. Maternal diabetes (pregestational and gestational) was evaluated as a potential effect modifier, and sensitivity analyses were conducted to evaluate whether receipt of a partial, single, or multiple course(s) of antenatal corticosteroids influenced results.
Results: A total of 4,429 women with 5,259 neonates met inclusion criteria: 3,716 (83.9%) women received antenatal corticosteroids and 713 (16.1%) did not. Of the 510 diabetic women (181 pregestational and 329 gestational), 439 (86.1%) received antenatal corticosteroids. Of the 3,919 nondiabetic women, 3,277 (83.6%) received antenatal corticosteroids. Antenatal corticosteroid exposure was not associated with respiratory distress syndrome or early death (adjusted relative risk [aRR] = 0.94, 95% confidence interval [CI]: 0.85-1.04), composite neonatal morbidity (aRR = 0.98, 95% CI: 0.89-1.07), or mechanical ventilation (aRR = 0.95, 95% CI: 0.86-1.05). There was no significant effect modification of maternal diabetes on the relationship between antenatal corticosteroids and neonatal outcomes (p > 0.05), and outcomes were similar in sensitivity analyses of partial, single, or multiple courses of corticosteroids.
Discussion: Antenatal corticosteroid administered to reduce preterm neonatal morbidity does not appear to have a differential association among women with diabetes compared with those without.
Key points: · Antenatal corticosteroids are used ubiquitously in women with and without diabetes.. · Maternal diabetes does not appear to modify the neonatal effect of antenatal corticosteroids.. · Larger studies of antenatal corticosteroids are needed to confirm our findings in diabetic women..
Thieme. All rights reserved.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Optimal timing of antenatal corticosteroid administration and preterm neonatal and early childhood outcomes.Am J Obstet Gynecol MFM. 2020 Feb;2(1):100077. doi: 10.1016/j.ajogmf.2019.100077. Epub 2019 Dec 17. Am J Obstet Gynecol MFM. 2020. PMID: 32905377 Free PMC article.
-
Antenatal Corticosteroids for the Prevention of Respiratory Distress Syndrome in Premature Twins.Obstet Gynecol. 2016 Sep;128(3):583-91. doi: 10.1097/AOG.0000000000001577. Obstet Gynecol. 2016. PMID: 27500336 Clinical Trial.
-
Gestational diabetes mellitus and late preterm birth: outcomes with and without antenatal corticosteroid exposure.Am J Obstet Gynecol MFM. 2024 Mar;6(3):101268. doi: 10.1016/j.ajogmf.2023.101268. Epub 2024 Jan 18. Am J Obstet Gynecol MFM. 2024. PMID: 38242498
-
Antenatal corticosteroids in preterm small-for-gestational age infants: a systematic review and meta-analysis.Am J Obstet Gynecol MFM. 2020 Nov;2(4):100215. doi: 10.1016/j.ajogmf.2020.100215. Epub 2020 Aug 17. Am J Obstet Gynecol MFM. 2020. PMID: 33345924 Free PMC article.
-
Antenatal corticosteroids after 34 weeks' gestation: Do we have the evidence?Semin Fetal Neonatal Med. 2019 Jun;24(3):189-196. doi: 10.1016/j.siny.2019.03.001. Epub 2019 Apr 2. Semin Fetal Neonatal Med. 2019. PMID: 31029555 Review.
Cited by
-
The effect of time interval between antenatal corticosteroid administration and delivery on outcomes in late preterm neonates born to mothers with diabetes: a retrospective cohort study.Front Pediatr. 2023 Aug 25;11:1239977. doi: 10.3389/fped.2023.1239977. eCollection 2023. Front Pediatr. 2023. PMID: 37691770 Free PMC article.
-
Maternal and neonatal outcomes following antenatal corticosteroids in pregnancies complicated by diabetes: a scoping review.AJOG Glob Rep. 2024 Nov 2;4(4):100416. doi: 10.1016/j.xagr.2024.100416. eCollection 2024 Nov. AJOG Glob Rep. 2024. PMID: 39650740 Free PMC article.
-
Time Interval From Early-Term Antenatal Corticosteroids Administration to Delivery and the Impact on Neonatal Outcomes.Front Pediatr. 2022 Apr 11;10:836220. doi: 10.3389/fped.2022.836220. eCollection 2022. Front Pediatr. 2022. PMID: 35479760 Free PMC article.
-
Antenatal corticosteroids in specific groups at risk of preterm birth: a systematic review.BMJ Open. 2023 Sep 22;13(9):e065070. doi: 10.1136/bmjopen-2022-065070. BMJ Open. 2023. PMID: 37739474 Free PMC article.
References
-
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep 2017;66(01):1 - PubMed
-
- Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 1995; 173(01 ):322–335 - PubMed
-
- Neilson JP. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Obstet Gynecol 2007;109(01 ):189–190 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD053118/HD/NICHD NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous