Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials
- PMID: 32718020
- PMCID: PMC7432953
- DOI: 10.3390/ijms21155224
Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials
Abstract
The ongoing pandemic of coronavirus disease-2019 (COVID-19) is being caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The disease continues to present significant challenges to the health care systems around the world. This is primarily because of the lack of vaccines to protect against the infection and the lack of highly effective therapeutics to prevent and/or treat the illness. Nevertheless, researchers have swiftly responded to the pandemic by advancing old and new potential therapeutics into clinical trials. In this review, we summarize potential anti-COVID-19 therapeutics that block the early stage of the viral life cycle. The review presents the structures, mechanisms, and reported results of clinical trials of potential therapeutics that have been listed in clinicaltrials.gov. Given the fact that some of these therapeutics are multi-acting molecules, other relevant mechanisms will also be described. The reviewed therapeutics include small molecules and macromolecules of sulfated polysaccharides, polypeptides, and monoclonal antibodies. The potential therapeutics target viral and/or host proteins or processes that facilitate the early stage of the viral infection. Frequent targets are the viral spike protein, the host angiotensin converting enzyme 2, the host transmembrane protease serine 2, and clathrin-mediated endocytosis process. Overall, the review aims at presenting update-to-date details, so as to enhance awareness of potential therapeutics, and thus, to catalyze their appropriate use in combating the pandemic.
Keywords: ACE2; COVID-19; SARS-CoV-2; TMPRSS2; coronavirus; endocytosis; spike protein; viral entry; viral fusion.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
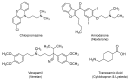
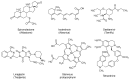


References
-
- World Health Organization [(accessed on 31 May 2020)]; Available online: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

