Effect of Low-dose and Standard-dose Aspirin on PGE2 Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial
- PMID: 32718943
- PMCID: PMC7541643
- DOI: 10.1158/1940-6207.CAPR-20-0216
Effect of Low-dose and Standard-dose Aspirin on PGE2 Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial
Abstract
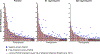
Low-dose aspirin is recommended by the U.S. Preventive Services Task Force for primary prevention of colorectal cancer in certain individuals. However, broader implementation will require improved precision prevention approaches to identify those most likely to benefit. The major urinary metabolite of PGE2, 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid (PGE-M), is a biomarker for colorectal cancer risk, but it is unknown whether PGE-M is modifiable by aspirin in individuals at risk for colorectal cancer. Adults (N = 180) who recently underwent adenoma resection and did not regularly use aspirin or NSAIDs were recruited to a double-blind, placebo-controlled, randomized trial of aspirin at 81 or 325 mg/day for 8-12 weeks. The primary outcome was postintervention change in urinary PGE-M as measured by LC/MS. A total of 169 participants provided paired urine samples for analysis. Baseline PGE-M excretion was 15.9 ± 14.6 (mean ± S.D, ng/mg creatinine). Aspirin significantly reduced PGE-M excretion (-4.7 ± 14.8) compared with no decrease (0.8 ± 11.8) in the placebo group (P = 0.015; mean duration of treatment = 68.9 days). Aspirin significantly reduced PGE-M levels in participants receiving either 81 (-15%; P = 0.018) or 325 mg/day (-28%; P < 0.0001) compared with placebo. In 40% and 50% of the individuals randomized to 81 or 325 mg/day aspirin, respectively, PGE-M reduction reached a threshold expected to prevent recurrence in 10% of individuals. These results support that aspirin significantly reduces elevated levels of PGE-M in those at increased colorectal cancer risk to levels consistent with lower risk for recurrent neoplasia and underscore the potential utility of PGE-M as a precision chemoprevention biomarker. The ASPIRED trial is registered as NCT02394769.
©2020 American Association for Cancer Research.
Figures


References
-
- Kortz L, Dorow J, Ceglarek U. Liquid chromatography-tandem mass spectrometry for the analysis of eicosanoids and related lipids in human biological matrices: a review. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2014;964:1–11 doi 10.1016/j.jchromb.2014.01.046. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

