Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study
- PMID: 32719045
- PMCID: PMC7456552
- DOI: 10.1136/annrheumdis-2020-218479
Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study
Abstract
Objectives: To prospectively investigate in patients with severe COVID-19-associated cytokine storm syndrome (CSS) whether an intensive course of glucocorticoids with or without tocilizumab accelerates clinical improvement, reduces mortality and prevents invasive mechanical ventilation, in comparison with a historic control group of patients who received supportive care only.
Methods: From 1 April 2020, patients with COVID-19-associated CSS, defined as rapid respiratory deterioration plus at least two out of three biomarkers with important elevations (C-reactive protein >100 mg/L; ferritin >900 µg/L; D-dimer >1500 µg/L), received high-dose intravenous methylprednisolone for 5 consecutive days (250 mg on day 1 followed by 80 mg on days 2-5). If the respiratory condition had not improved sufficiently (in 43%), the interleukin-6 receptor blocker tocilizumab (8 mg/kg body weight, single infusion) was added on or after day 2. Control patients with COVID-19-associated CSS (same definition) were retrospectively sampled from the pool of patients (n=350) admitted between 7 March and 31 March, and matched one to one to treated patients on sex and age. The primary outcome was ≥2 stages of improvement on a 7-item WHO-endorsed scale for trials in patients with severe influenza pneumonia, or discharge from the hospital. Secondary outcomes were hospital mortality and mechanical ventilation.
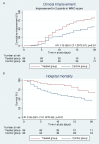
Results: At baseline all patients with COVID-19 in the treatment group (n=86) and control group (n=86) had symptoms of CSS and faced acute respiratory failure. Treated patients had 79% higher likelihood on reaching the primary outcome (HR: 1.8; 95% CI 1.2 to 2.7) (7 days earlier), 65% less mortality (HR: 0.35; 95% CI 0.19 to 0.65) and 71% less invasive mechanical ventilation (HR: 0.29; 95% CI 0.14 to 0.65). Treatment effects remained constant in confounding and sensitivity analyses.
Conclusions: A strategy involving a course of high-dose methylprednisolone, followed by tocilizumab if needed, may accelerate respiratory recovery, lower hospital mortality and reduce the likelihood of invasive mechanical ventilation in COVID-19-associated CSS.
Keywords: biological therapy; cytokines; epidemiology; glucocorticoids.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SR reports personal fees from AbbVie, personal fees from Eli Lilly, grants and personal fees from MSD, personal fees from Novartis, personal fees from UCB, personal fees from Sanofi, outside the submitted work. RLMM reports personal fees from Boehringer Ingelheim, personal fees from Roche, personal fees from Galapagos, outside the submitted work. CMC is a clinical trial investigator for a study sponsored by Lilly and was a subinvestigator for a study sponsored by GSK. CvD reports personal fees from Novartis, personal fees from Roche, outside the submitted work. TD reports grants from Adrenomed, grants from Inotrem, grants from Roche, grants from Shionogi and Co, other from CASTOR, outside the submitted work. MG reports personal fees from Roche, personal fees from MSD, outside the submitted work. MdK reports personal fees from ALK, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Sanofi Genzyme, outside the submitted work. ML reports grants from AstraZeneca, grants from Pfizer, personal fees from Roche, outside the submitted work. RP reports grants and personal fees from Pfizer, grants and personal fees from AbbVie, outside the submitted work. RL reports personal fees from AbbVie, personal fees from BMS, personal fees from Galapagos, personal fees from Gilead, personal fees from Jansen, personal fees from Novartis, personal fees from Pfizer, personal fees from Roche, personal fees from UCB, outside the submitted work; and owner and director of Rheumatology Consultancy, a company that provides consultancy and read services for clinical trials.
Figures
Comment in
-
To immunosuppress: whom, when and how? That is the question with COVID-19.Ann Rheum Dis. 2020 Sep;79(9):1129-1131. doi: 10.1136/annrheumdis-2020-218694. Epub 2020 Aug 4. Ann Rheum Dis. 2020. PMID: 32753413 Free PMC article. No abstract available.
-
Response to: 'High dosage of Methylprednisolone as a rescue, second-line treatment in COVID-19 patients who failed to respond to Tocilizumab' by Conticini et al.Ann Rheum Dis. 2022 Oct;81(10):e203. doi: 10.1136/annrheumdis-2020-218788. Epub 2020 Aug 19. Ann Rheum Dis. 2022. PMID: 32816700 No abstract available.
-
High dosage of methylprednisolone as a rescue, second-line treatment in COVID-19 patients who failed to respond to tocilizumab.Ann Rheum Dis. 2022 Oct;81(10):e202. doi: 10.1136/annrheumdis-2020-218761. Epub 2020 Aug 19. Ann Rheum Dis. 2022. PMID: 32816701 No abstract available.
-
Response to: 'Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'' by Charles.Ann Rheum Dis. 2023 May;82(5):e109. doi: 10.1136/annrheumdis-2021-220001. Epub 2021 Feb 15. Ann Rheum Dis. 2023. PMID: 33589439 No abstract available.
-
Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'.Ann Rheum Dis. 2023 May;82(5):e108. doi: 10.1136/annrheumdis-2021-219986. Epub 2021 Feb 15. Ann Rheum Dis. 2023. PMID: 33589440 No abstract available.
-
Response to: 'Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'' by De Santis et al.Ann Rheum Dis. 2023 May;82(5):e112. doi: 10.1136/annrheumdis-2021-220077. Epub 2021 Feb 16. Ann Rheum Dis. 2023. PMID: 33593737 No abstract available.
-
Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'.Ann Rheum Dis. 2023 May;82(5):e111. doi: 10.1136/annrheumdis-2021-220044. Epub 2021 Feb 16. Ann Rheum Dis. 2023. PMID: 33593740 No abstract available.
-
Response to: 'Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'' by Charles.Ann Rheum Dis. 2023 May;82(5):e110. doi: 10.1136/annrheumdis-2021-220164. Epub 2021 Mar 3. Ann Rheum Dis. 2023. PMID: 33658235 No abstract available.
-
Response to: 'Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'' by Kaklamanos et al.Ann Rheum Dis. 2023 Jun;82(6):e135. doi: 10.1136/annrheumdis-2021-220474. Epub 2021 Apr 26. Ann Rheum Dis. 2023. PMID: 33903095 No abstract available.
-
Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'.Ann Rheum Dis. 2023 Jun;82(6):e134. doi: 10.1136/annrheumdis-2021-220411. Epub 2021 Apr 26. Ann Rheum Dis. 2023. PMID: 33903096 No abstract available.
-
Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study'.Ann Rheum Dis. 2023 Jul;82(7):e153. doi: 10.1136/annrheumdis-2021-220771. Epub 2021 Jun 23. Ann Rheum Dis. 2023. PMID: 34162593 No abstract available.
-
Response to: 'Correspondence on 'Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19- associated cytokine storm syndrome: results of the CHIC study'' by Klopfenstein et al.Ann Rheum Dis. 2023 Jul;82(7):e154. doi: 10.1136/annrheumdis-2021-220787. Epub 2021 Jun 23. Ann Rheum Dis. 2023. PMID: 34162596 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous