Hedgehog signaling is necessary and sufficient to mediate craniofacial plasticity in teleosts
- PMID: 32719137
- PMCID: PMC7431006
- DOI: 10.1073/pnas.1921856117
Hedgehog signaling is necessary and sufficient to mediate craniofacial plasticity in teleosts
Abstract
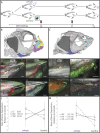
Phenotypic plasticity, the ability of a single genotype to produce multiple phenotypes under different environmental conditions, is critical for the origins and maintenance of biodiversity; however, the genetic mechanisms underlying plasticity as well as how variation in those mechanisms can drive evolutionary change remain poorly understood. Here, we examine the cichlid feeding apparatus, an icon of both prodigious evolutionary divergence and adaptive phenotypic plasticity. We first provide a tissue-level mechanism for plasticity in craniofacial shape by measuring rates of bone deposition within functionally salient elements of the feeding apparatus in fishes forced to employ alternate foraging modes. We show that levels and patterns of phenotypic plasticity are distinct among closely related cichlid species, underscoring the evolutionary potential of this trait. Next, we demonstrate that hedgehog (Hh) signaling, which has been implicated in the evolutionary divergence of cichlid feeding architecture, is associated with environmentally induced rates of bone deposition. Finally, to demonstrate that Hh levels are the cause of the plastic response and not simply the consequence of producing more bone, we use transgenic zebrafish in which Hh levels could be experimentally manipulated under different foraging conditions. Notably, we find that the ability to modulate bone deposition rates in different environments is dampened when Hh levels are reduced, whereas the sensitivity of bone deposition to different mechanical demands increases with elevated Hh levels. These data advance a mechanistic understanding of phenotypic plasticity in the teleost feeding apparatus and in doing so contribute key insights into the origins of adaptive morphological radiations.
Keywords: craniofacial; ecodevo; flexible stem; hedgehog signaling; phenotypic plasticity.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Bradshaw A. D., Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155 (1965).
-
- Schlichting C. D., Pigliucci M., Phenotypic Evolution. A Reaction Norm Perspective, (Sinauer Associates, Sunderland, MA, 1998).
-
- Pigliucci M., Evolution of phenotypic plasticity: Where are we going now? Trends Ecol. Evol. 20, 481–486 (2005). - PubMed
-
- Luo L., Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601–635 (2002). - PubMed
-
- Flück M., Hoppeler H., “Molecular basis of skeletal muscle plasticity-from gene to form and function” in Reviews of Physiology, Biochemistry and Pharmacology, (Springer, Berlin, Germany, 2003), Vol. 146, pp. 159–216. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

