Leukocidins and the Nuclease Nuc Prevent Neutrophil-Mediated Killing of Staphylococcus aureus Biofilms
- PMID: 32719153
- PMCID: PMC7504955
- DOI: 10.1128/IAI.00372-20
Leukocidins and the Nuclease Nuc Prevent Neutrophil-Mediated Killing of Staphylococcus aureus Biofilms
Abstract
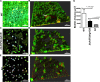
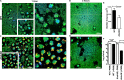
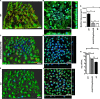
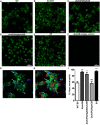
Bacterial biofilms are linked with chronic infections and have properties distinct from those of planktonic, single-celled bacteria. The virulence mechanisms associated with Staphylococcus aureus biofilms are becoming better understood. Human neutrophils are critical for the innate immune response to S. aureus infection. Here, we describe two virulence strategies that converge to promote the ability of S. aureus biofilms to evade killing by neutrophils. Specifically, we show that while neutrophils exposed to S. aureus biofilms produce extracellular traps (NETs) and phagocytose bacteria, both mechanisms are inefficient in clearance of the biofilm biomass. This is attributed to the leukocidin LukAB, which promotes S. aureus survival during phagocytosis. We also show that the persistence of biofilm bacteria trapped in NETs is facilitated by S. aureus nuclease (Nuc)-mediated degradation of NET DNA. This study describes key aspects of the interaction between primary human neutrophils and S. aureus biofilms and provides insight into how S. aureus evades the neutrophil response to cause persistent infections.
Keywords: NETs; S. aureus; biofilms; neutrophil; phagocytosis.
Copyright © 2020 American Society for Microbiology.
Figures



References
-
- Chapman JR, Balasubramanian D, Tam K, Askenazi M, Copin R, Shopsin B, Torres VJ, Ueberheide BM. 2017. Using quantitative spectrometry to understand the influence of genetics and nutritional perturbations on the virulence potential of Staphylococcus aureus. Mol Cell Proteomics 16:S15–S28. doi:10.1074/mcp.O116.065581. - DOI - PMC - PubMed
-
- Ammons MCB, Tripet BP, Carlson RP, Kirker KR, Gross MA, Stanisich JJ, Copié V. 2014. Quantitative NMR metabolite profiling of methicillin-resistant and methicillin-susceptible Staphylococcus aureus discriminates between biofilm and planktonic phenotypes. J Proteome Res 3:2973–2985. doi:10.1021/pr500120c. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

