Allergic bronchopulmonary aspergillosis
- PMID: 32719226
- PMCID: PMC7602921
- DOI: 10.4103/ijmr.IJMR_1187_19
Allergic bronchopulmonary aspergillosis
Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is an inflammatory disease caused by immunologic reactions initiated against Aspergillus fumigatus colonizing the airways of patients with asthma and cystic fibrosis. The common manifestations include treatment-resistant asthma, transient and fleeting pulmonary opacities and bronchiectasis. It is believed that globally there are about five million cases of ABPA, with India alone accounting for about 1.4 million cases. The occurrence of ABPA among asthmatic patients in special clinics may be as high as 13 per cent. Thus, a high degree of suspicion for ABPA should be entertained while treating a patient with bronchial asthma, particularly in specialized clinics. Early diagnosis and appropriate treatment can delay (or even prevent) the onset of bronchiectasis, which suggests that all patients of bronchial asthma should be screened for ABPA, especially in chest clinics. The current review summarizes the recent advances in the pathogenesis, diagnosis and management of ABPA.
Keywords: Allergic bronchopulmonary aspergillosis; Aspergillus; allergic bronchopulmonary mycosis; asthma; azole; azole - cystic fibrosis - glucocorticoids; cystic fibrosis; glucocorticoids.
Conflict of interest statement
None
Figures


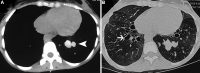
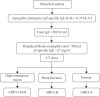

References
-
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–7. - PubMed
-
- Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135:805–26. - PubMed
-
- Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850–73. - PubMed
-
- Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(Suppl 3):S225–64. - PubMed
-
- Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in North India. Chest. 2006;130:442–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
