Molecular engineering of safe and efficacious oral basal insulin
- PMID: 32719315
- PMCID: PMC7385171
- DOI: 10.1038/s41467-020-17487-9
Molecular engineering of safe and efficacious oral basal insulin
Erratum in
-
Author Correction: Molecular engineering of safe and efficacious oral basal insulin.Nat Commun. 2020 Aug 20;11(1):4232. doi: 10.1038/s41467-020-18106-3. Nat Commun. 2020. PMID: 34244486 Free PMC article. No abstract available.
Abstract
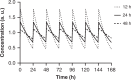
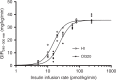
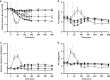
Recently, the clinical proof of concept for the first ultra-long oral insulin was reported, showing efficacy and safety similar to subcutaneously administered insulin glargine. Here, we report the molecular engineering as well as biological and pharmacological properties of these insulin analogues. Molecules were designed to have ultra-long pharmacokinetic profile to minimize variability in plasma exposure. Elimination plasma half-life of ~20 h in dogs and ~70 h in man is achieved by a strong albumin binding, and by lowering the insulin receptor affinity 500-fold to slow down receptor mediated clearance. These insulin analogues still stimulate efficient glucose disposal in rats, pigs and dogs during constant intravenous infusion and euglycemic clamp conditions. The albumin binding facilitates initial high plasma exposure with a concomitant delay in distribution to peripheral tissues. This slow appearance in the periphery mediates an early transient hepato-centric insulin action and blunts hypoglycaemia in dogs in response to overdosing.
Conflict of interest statement
All authors except M.C.M. and A.D.C. are present or past employees of Novo Nordisk and hold equity in the company. M.C.M. and A.D.C. received financial support from Novo Nordisk. Insulin analogues and formulations described in this article are covered by Novo Nordisk’s patents and patent applications. A.D.C. reports grants, personal fees, and other from Metavention, Zafgen, and Abvance; grants and/or personal fees from Thetis Pharmaceuticals, Boston Scientific, Novo Nordisk, vTv Therapeutics, Merck, Eli Lilly, and Galvani Bioelectronics; personal fees from California Institute for Biomedical Research (Calibr) and MedImmune; personal fees and other from Fractyl, Biocon and Sensulin Labs.
Figures



References
-
- Berger, M. in Frontiers in Insulin Pharmacology (eds Berger, M. & Gries, F.) 144–148 (Plenum, Stuttgart, 1993).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

