Bioactive DNA from extracellular vesicles and particles
- PMID: 32719324
- PMCID: PMC7385258
- DOI: 10.1038/s41419-020-02803-4
Bioactive DNA from extracellular vesicles and particles
Abstract
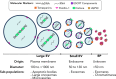
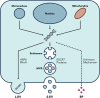
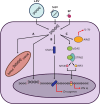
Extracellular vesicles (EVs) and particles (EPs) have recently emerged as active carriers of molecular biomarkers and mediators of intercellular communication. While most investigations have focused exclusively on the protein, lipid and RNA constituents of these extracellular entities, EV/EP DNA remains poorly understood, despite DNA being found in association with virtually all EV/EP populations. The functional potential of EV/EP DNA has been proposed in a number of pathological states, including malignancies and autoimmune diseases. Moreover, the effectiveness of cell-free DNA as the biomarker of choice in emerging liquid biopsy applications highlights the role that EV/EP DNA may play as a novel disease biomarker. In this review, we provide a comprehensive overview of EV/EP DNA studies conducted to date, with a particular focus on the roles of EV/EP DNA as a functional mediator and molecular biomarker in various pathologic states. We also review what is currently known about the origins, structure, localisation and distribution of EV/EP DNA, highlighting current controversies as well as opportunities for future investigation.
Conflict of interest statement
S.V.B. provides consultation for Bristol-Myers Squibb, receives institutional research support from Nektar Therapeutics, is a co-inventor on a patent relating to mutation-based ctDNA detection technology that has been licensed to Roche Molecular Diagnostics and on patent applications relating to methylation-based ctDNA detection technology, and is co-founder and provides consultation for DNAMx, Inc.
Figures
References
-
- Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer17, 223–238 (2017). - PubMed
-
- Cescon, D. W., Bratman, S. V., Chan, S. M. & Siu, L. L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer1, 276–290 (2020). - PubMed
-
- Hawes, M. C., Wen, F. & Elquza, E. Extracellular DNA: a bridge to cancer. Cancer Res.75, 4260–4264 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

