Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial
- PMID: 32719455
- PMCID: PMC8027015
- DOI: 10.1038/s41422-020-0375-9
Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial
Abstract
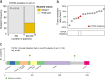
Triple-negative breast cancer (TNBC) is a highly heterogeneous disease, and molecular subtyping may result in improved diagnostic precision and targeted therapies. Our previous study classified TNBCs into four subtypes with putative therapeutic targets. Here, we conducted the FUTURE trial (ClinicalTrials.gov identifier: NCT03805399), a phase Ib/II subtyping-based and genomic biomarker-guided umbrella trial, to evaluate the efficacy of these targets. Patients with refractory metastatic TNBC were enrolled and stratified by TNBC subtypes and genomic biomarkers, and assigned to one of these seven arms: (A) pyrotinib with capecitabine, (B) androgen receptor inhibitor with CDK4/6 inhibitor, (C) anti PD-1 with nab-paclitaxel, (D) PARP inhibitor included, (E) and (F) anti-VEGFR included, or (G) mTOR inhibitor with nab-paclitaxel. The primary end point was the objective response rate (ORR). We enrolled 69 refractory metastatic TNBC patients with a median of three previous lines of therapy (range, 1-8). Objective response was achieved in 20 (29.0%, 95% confidence interval (CI): 18.7%-41.2%) of the 69 intention-to-treat (ITT) patients. Our results showed that immunotherapy (arm C), in particular, achieved the highest ORR (52.6%, 95% CI: 28.9%-75.6%) in the ITT population. Arm E demonstrated favorable ORR (26.1%, 95% CI: 10.2%-48.4% in the ITT population) but with more high grade (≥ 3) adverse events. Somatic mutations of TOP2A and CD8 immunohistochemical score may have the potential to predict immunotherapy response in the immunomodulatory subtype of TNBC. In conclusion, the phase Ib/II FUTURE trial suggested a new concept for TNBC treatment, demonstrating the clinical benefit of subtyping-based targeted therapy for refractory metastatic TNBC.
Conflict of interest statement
Xiaoyu Zhu and Jianjun Zou are employees of Jiangsu Hengrui Medicine Co, Ltd. No other potential conflicts of interest were reported.
Figures



References
-
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. - PubMed
-
- Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. - PubMed
-
- Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389:2430–2442. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

