Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin
- PMID: 32719487
- PMCID: PMC8176964
- DOI: 10.1038/s41591-020-0985-2
Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin
Abstract
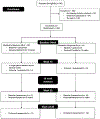
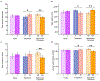
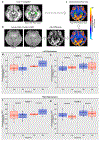
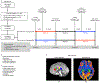
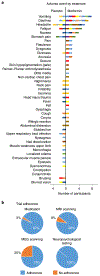
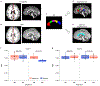
We asked whether pharmacological stimulation of endogenous neural precursor cells (NPCs) may promote cognitive recovery and brain repair, focusing on the drug metformin, in parallel rodent and human studies of radiation injury. In the rodent cranial radiation model, we found that metformin enhanced the recovery of NPCs in the dentate gyrus, with sex-dependent effects on neurogenesis and cognition. A pilot double-blind, placebo-controlled crossover trial was conducted (ClinicalTrials.gov, NCT02040376) in survivors of pediatric brain tumors who had been treated with cranial radiation. Safety, feasibility, cognitive tests and MRI measures of white matter and the hippocampus were evaluated as endpoints. Twenty-four participants consented and were randomly assigned to complete 12-week cycles of metformin (A) and placebo (B) in either an AB or BA sequence with a 10-week washout period at crossover. Blood draws were conducted to monitor safety. Feasibility was assessed as recruitment rate, medication adherence and procedural adherence. Linear mixed modeling was used to examine cognitive and MRI outcomes as a function of cycle, sequence and treatment. We found no clinically relevant safety concerns and no serious adverse events associated with metformin. Sequence effects were observed for all cognitive outcomes in our linear mixed models. For the subset of participants with complete data in cycle 1, metformin was associated with better performance than placebo on tests of declarative and working memory. We present evidence that a clinical trial examining the effects of metformin on cognition and brain structure is feasible in long-term survivors of pediatric brain tumors and that metformin is safe to use and tolerable in this population. This pilot trial was not intended to test the efficacy of metformin for cognitive recovery and brain growth, but the preliminary results are encouraging and warrant further investigation in a large multicenter phase 3 trial.
Figures






References
-
- Miller FD & Kaplan DR Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell 10, 650–652 (2012). - PubMed
-
- Lazarini F & Lledo PM Is adult neurogenesis essential for olfaction? Trends Neurosci. 34, 20–30 (2011). - PubMed
-
- Wang J et al. Metformin activates an atypical PKC–CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11, 23–35 (2012). - PubMed
-
- Chow A & Morshead CM Cyclosporin A enhances neurogenesis in the dentate gyrus of the hippocampus. Stem Cell Res. 16, 79–87 (2016). - PubMed

