Profound and redundant functions of arcuate neurons in obesity development
- PMID: 32719538
- PMCID: PMC7687864
- DOI: 10.1038/s42255-020-0229-2
Profound and redundant functions of arcuate neurons in obesity development
Abstract
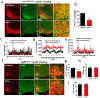
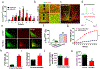
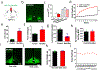
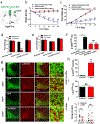
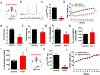
The current obesity epidemic faces a lack of mechanistic insights. It is known that the acute activity changes of a growing number of brain neurons rapidly alter feeding behaviour; however, how these changes translate to obesity development and the fundamental mechanism underlying brain neurons in controlling body weight remain elusive. Here, we show that chronic activation of hypothalamic arcuate GABAergic (GABA+), agouti-related protein (AgRP) neurons or arcuate non-AgRP GABA+ neurons leads to obesity, which is similar to the obese phenotype observed in ob/ob mice. Conversely, chronic inhibition of arcuate GABA+, but not AgRP, neurons reduces ageing-related weight gain and corrects ob/ob obesity. These results demonstrate that the modulation of Arc GABA+ neuron activity is a fundamental mechanism of body-weight regulation, and that arcuate GABA+ neurons are the major mediator of leptin action, with a profound and redundant role in obesity development.
Conflict of interest statement
Competing interests:
All authors declare no competing interests.
Figures



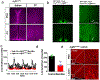
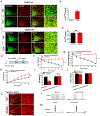
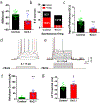
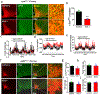





References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK109194/DK/NIDDK NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 DK114279/DK/NIDDK NIH HHS/United States
- R01 MH117089/MH/NIMH NIH HHS/United States
- R01 DK117281/DK/NIDDK NIH HHS/United States
- R01 DK114356/DK/NIDDK NIH HHS/United States
- P50 HD103555/HD/NICHD NIH HHS/United States
- R01 DK109934/DK/NIDDK NIH HHS/United States
- R01 DK101379/DK/NIDDK NIH HHS/United States
- R21 NS108091/NS/NINDS NIH HHS/United States
- R56 DK109194/DK/NIDDK NIH HHS/United States
- UM1 HG006348/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

