Antigen-Dependent T Cell Response to Neural Peptides After Human Ischemic Stroke
- PMID: 32719588
- PMCID: PMC7348665
- DOI: 10.3389/fncel.2020.00206
Antigen-Dependent T Cell Response to Neural Peptides After Human Ischemic Stroke
Abstract
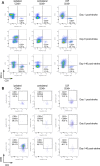
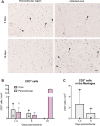
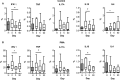
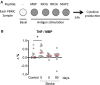
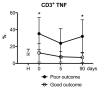
Ischemic stroke causes brain tissue damage and may release central nervous system (CNS)-specific peptides to the periphery. Neural antigen presentation in the lymphoid tissue could prime immune cells and result in adaptive immune response. However, autoimmune responses against neural antigens are not commonly uncovered after stroke. We studied the brain tissue of nine fatal stroke cases and the blood of a cohort of 13 patients and 11 controls. Flow cytometry carried out in three of the brain samples showed CD8 and CD4 T cells in the cerebrospinal fluid (CSF) of the ventricles in the patient deceased 1 day poststroke, T cells with an activated phenotype in the CSF of the patient that died at day 6, and T cells in the ischemic brain tissue in the patient deceased 140 days after stroke onset. Immunohistochemistry showed higher T cell numbers in the core of the lesion of the patient deceased 18 days post-stroke than in the patients deceased from 1 to 5 days post-stroke. In blood samples, we studied whether lymphocytes were primed in the periphery against neural antigens at sequential times (on admission, day 5, and day 90) after stroke. T lymphocytes of stroke patients produced IFN-γ and TNF-α and responded to MBP peptides by increasing their production of TNF-α and IL-10 at admission, but not at later time points. In contrast, IL-4 producing T cells showed progressive increases. Higher percentages of TNF-α producing T lymphocytes at admission were independently associated with poorer outcomes at 90 days. However, we did not detect T cell responses to neural-antigen stimulation 90 days post-stroke. Altogether the results suggest acute T cell priming in the periphery in acute stroke, T cell trafficking from the CSF to the ischemic brain tissue, and the existence of active mechanisms preventing autoreactivity.
Keywords: T-cell response; antigen-specificity; brain autopsy; brain infiltration; cytokine production; stroke.
Copyright © 2020 Miró-Mur, Urra, Ruiz-Jaén, Pedragosa, Chamorro and Planas.
Figures

References
-
- Albargothy N. J., Johnston D. A., MacGregor-Sharp M., Weller R. O., Verma A., Hawkes C. A., et al. . (2018). Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 136, 139–152. 10.1007/s00401-018-1862-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

