Mesenchymal Stem Cells: A New Piece in the Puzzle of COVID-19 Treatment
- PMID: 32719683
- PMCID: PMC7347794
- DOI: 10.3389/fimmu.2020.01563
Mesenchymal Stem Cells: A New Piece in the Puzzle of COVID-19 Treatment
Abstract
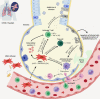
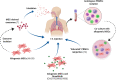
COVID-19 is a disease characterized by a strong inflammatory response in severe cases, which fails to respond to corticosteroid therapy. In the context of the current COVID-19 outbreak and the critical information gaps regarding the disease, several different therapeutic strategies are under investigation, including the use of stem cells. In the present manuscript, we provide an analysis of the rationale underlying the application of stem cells to manage COVID-19, and also a comprehensive compendium of the 69 clinical trials underway worldwide aiming to investigate the application of stem cells to treat COVID-19. Even though data are still scarce, it is already possible to observe the protagonism of China in testing mesenchymal stem cells (MSCs) for COVID-19. Furthermore, it is possible to determine that current efforts focus on the use of multiple infusions of high numbers of stem cells and derived products, as well as to acknowledge the positive results obtained by independent groups who publicized the therapeutic benefits provided by such therapies in 51 COVID-19 patients. In such a rapid-paced field, up-to-date systematic studies and meta-analysis will aid the scientific community to separate hype from hope and offer an unbiased position to the society and governments.
Keywords: COVID-19; SARS-CoV-2; clinical trials; mesenchymal stem cells; stem cell-therapy.
Copyright © 2020 Saldanha-Araujo, Melgaço Garcez, Silva-Carvalho and Carvalho.
Figures
Comment in
-
Commentary: Mesenchymal Stem Cells: A New Piece in the Puzzle of COVID-19 Treatment.Front Immunol. 2021 Apr 29;12:682195. doi: 10.3389/fimmu.2021.682195. eCollection 2021. Front Immunol. 2021. PMID: 33995424 Free PMC article. No abstract available.
References
-
- Lin Z, Gao Q, Qian F, Jinlian M, Lishi Z, Tian C, et al. The nucleocapsid protein of SARS-CoV-2 abolished pluripotency in human induced pluripotent stem cells. Biorxiv. (2020). [Epub ahead of print]. 10.1101/2020.03.26.010694. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

