Metal Organic Framework - Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions
- PMID: 32719772
- PMCID: PMC7350925
- DOI: 10.3389/fchem.2020.00534
Metal Organic Framework - Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions
Abstract
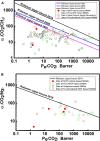
Gas separation and purification using polymeric membranes is a promising technology that constitutes an energy-efficient and eco-friendly process for large scale integration. However, pristine polymeric membranes typically suffer from the trade-off between permeability and selectivity represented by the Robeson's upper bound. Mixed matrix membranes (MMMs) synthesized by the addition of porous nano-fillers into polymer matrices, can enable a simultaneous increase in selectivity and permeability. Among the various porous fillers, metal-organic frameworks (MOFs) are recognized in recent days as a promising filler material for the fabrication of MMMs. In this article, we review representative examples of MMMs prepared by dispersion of MOFs into polymer matrices or by deposition on the surface of polymeric membranes. Addition of MOFs into other continuous phases, such as ionic liquids, are also included. CO2 separation from hydrocarbons, H2, N2, and the like is emphasized. Hybrid fillers based on composites of MOFs with other nanomaterials, e.g., of MOF/GO, MOF/CNTs, and functionalized MOFs, are also presented and discussed. Synergetic effects and the result of interactions between filler/matrix and filler/filler are reviewed, and the impact of filler and matrix types and compositions, filler loading, surface area, porosity, pore sizes, and surface functionalities on tuning permeability are discoursed. Finally, selectivity, thermal, chemical, and mechanical stability of the resulting MMMs are analyzed. The review concludes with a perspective of up-scaling of such systems for CO2 separation, including an overview of the most promising MMM systems.
Keywords: CO2; MOF; membranes; mixture; permeability; polymers; selectivity; separation.
Copyright © 2020 Muthukumaraswamy Rangaraj, Wahab, Reddy, Kakosimos, Abdalla, Favvas, Reinalda, Geuzebroek, Abdala and Karanikolos.
Figures



References
-
- Abdulrahman R. K., Sebastine I. M. (2013). Natural gas sweetening process simulation and optimization: a case study of khurmala field in iraqi kurdistan region. J. Nat. Gas Sci. Eng. 14, 116–120. 10.1016/j.jngse.2013.06.005 - DOI
-
- Adewole J. K., Ahmad A. L., Ismail S., Leo C. P. (2013). Current challenges in membrane separation of CO2 from natural gas: a review. Int. J. Greenhouse Gas Control 17, 46–65. 10.1016/j.ijggc.2013.04.012 - DOI
-
- Afarani H. T., Sadeghi M., Moheb A. (2018). The gas separation performance of polyurethane–zeolite mixed matrix membranes. Adv. Polym. Technol. 37, 339–348. 10.1002/adv.21672 - DOI
-
- Ahmadi Feijani E., Tavasoli A., Mahdavi H. (2015). Improving gas separation performance of poly(vinylidene fluoride) based mixed matrix membranes containing metal–organic frameworks by chemical Modification. Ind. Eng. Chem. Res 54, 12124–12134. 10.1021/acs.iecr.5b02549 - DOI
-
- Alomair A. A., Al-Jubouri S. M., Holmes S. M. (2015). A novel approach to fabricate zeolite membranes for pervaporation processes. J. Mater. Chem. A 3, 9799–9806. 10.1039/C5TA00124B - DOI
Publication types
LinkOut - more resources
Full Text Sources

