Lipid-Based Nanovesicles for Simultaneous Intracellular Delivery of Hydrophobic, Hydrophilic, and Amphiphilic Species
- PMID: 32719782
- PMCID: PMC7350901
- DOI: 10.3389/fbioe.2020.00690
Lipid-Based Nanovesicles for Simultaneous Intracellular Delivery of Hydrophobic, Hydrophilic, and Amphiphilic Species
Abstract
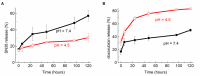
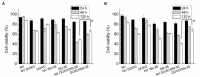
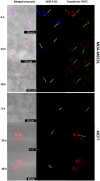
Lipid nanovesicles (NVs) are the first nanoformulation that entered the clinical use in oncology for the treatment of solid tumors. They are indeed versatile systems which can be loaded with either hydrophobic or hydrophilic molecules, for both imaging and drug delivery, and with high biocompatibility, and limited immunogenicity. In the present work, NVs with a lipid composition resembling that of natural vesicles were prepared using the ultrasonication method. The NVs were successfully loaded with fluorophores molecules (DOP-F-DS and a fluorescent protein), inorganic nanoparticles (quantum dots and magnetic nanoparticles), and anti-cancer drugs (SN-38 and doxorubicin). The encapsulation of such different molecules showed the versatility of the developed systems. The size of the vesicles varied from 100 up to 300 nm depending on the type of loaded species, which were accommodated either into the lipid bilayer or into the aqueous core according to their hydrophobic or hydrophilic nature. Viability assays were performed on cellular models of breast cancer (MCF-7 and MDA-MB-231). Results showed that NVs with encapsulated both drugs simultaneously led to a significant reduction of the cellular activity (up to 22%) compared to the free drugs or to the NVs encapsulated with only one drug. Lipidomic analysis suggested that the mechanism of action of the drugs is the same, whether they are free or encapsulated, but administration of the drugs by means of nanovesicles is more efficient in inducing cellular damage, likely because of a quicker internalization and a sustained release. This study confirms the versatility and the potential of lipid NVs for cancer treatment, as well as the validity of the ultrasound preparation method for their preparation.
Keywords: SN-38; breast cancer; doxorubicin; lipidomic analysis; nanoparticle; nanovesicle.
Copyright © 2020 Zacheo, Bizzarro, Blasi, Piccirillo, Cardone, Gigli, Ragusa and Quarta.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

