The AO trauma CPP bone infection registry: Epidemiology and outcomes of Staphylococcus aureus bone infection
- PMID: 32720352
- PMCID: PMC7749080
- DOI: 10.1002/jor.24804
The AO trauma CPP bone infection registry: Epidemiology and outcomes of Staphylococcus aureus bone infection
Abstract
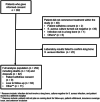
Bone infection represents a serious complication of orthopedic surgery and Staphylococcus aureus is the most common pathogen. To improve the understanding of host-pathogen interaction, we developed a biospecimen registry (AO Trauma CPP Bone Infection Registry) to collect clinical data, bacterial isolates, and serum from patients with S. aureus bone infection. A prospective multinational registry with a 12-month follow-up was created to include adult patients (18 years or older) with culture-confirmed S. aureus infection in long bones after fracture fixation or arthroplasty. Baseline patient attributes and details on infections and treatments were recorded. Blood and serum samples were obtained at baseline, 6, and 12 months. Patient-reported outcomes were collected at 1, 6, and 12 months. Clinical outcomes were recorded. Two hundred and ninety-two patients with fracture-related infection (n = 157, 53.8%), prosthetic joint infection (n = 86, 29.5%), and osteomyelitis (n = 49, 16.8%) were enrolled. Methicillin-resistant S. aureus was detected in 82 patients (28.4%), with the highest proportion found among patients from North American sites (n = 39, 48.8%) and the lowest from Central European sites (n = 18, 12.2%). Patient outcomes improved at 6 and 12 months in comparison to baseline. The SF-36 physical component summary mean (95% confidence interval) score, however, did not reach 50 at 12 months. The cure rate at the end of the study period was 62.1%. Although patients improved with treatment, less than two-thirds were cured in 1 year. At 12-month follow-up, patient-reported outcome scores were worse for patients with methicillin-resistant S. aureus infections.
Keywords: MRSA; Staphylococcus aureus; bone infection registry; fracture-related infection; implant-related infection.
© 2020 The Authors. Journal of Orthopaedic Research® published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
SLK: Grant‐AO Trauma CPP Bone Infection—PI, DePuy Synthes—in kind research support, NIH (P50 AR072000), NCATS CTSA Grant # 1UL1TR002649, PCORI and Journal Editor—Sage Publications. MM: Research support from AO Trauma. WJM: received research support from AO Trauma, is a consultant for Depuy Synthes and member of the speakers bureau of Zimmer Biomet. MB: Franchise Medical Director, Preclinical Clinical Medical Trauma CMF Biomaterials, Depuy Synthes. FL: Research support from AO Trauma, Journal Deputy Editor Sage Publication, Depuy Synthes. KS, XZ, CE, MM, JS, MS, MN, JB, DS, KY, BQ, YL: none reported.
Figures
References
-
- Saeed K, McLaren AC, Schwarz EM, et al. 2018 International Consensus Meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res. 2019;37:1007‐1017. - PubMed
-
- Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49:511‐522. - PubMed
-
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic‐joint infections. N Engl J Med. 2004;351:1645‐1654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

