Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives
- PMID: 32720441
- PMCID: PMC7756885
- DOI: 10.1002/anie.202008838
Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives
Abstract
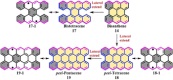
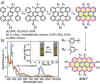
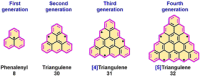
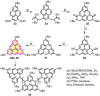
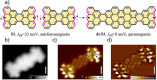
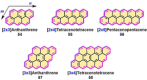
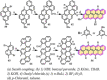
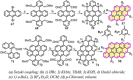
Experimental and theoretical investigations have revealed that the chemical and physical properties of graphene are crucially determined by their topological structures. Therefore, the atomically precise synthesis of graphene nanostructures is essential. A particular example is graphene nanostructures with zigzag-edged structures, which exhibit unique (opto)electronic and magnetic properties owing to their spin-polarized edge state. Recent progress in the development of synthetic methods and strategies as well as characterization methods has given access to this class of unprecedented graphene nanostructures, which used to be purely molecular objectives in theoretical chemistry. Thus, clear insight into the structure-property relationships has become possible as well as new applications in organic carbon-based electronic and spintronic devices. In this Minireview, we discuss the recent progress in the controlled synthesis of zigzag-edged graphene nanostructures with different topologies through a bottom-up synthetic strategy.
Keywords: bottom-up synthesis; doping; graphene nanoribbons; magnetism; nanographenes.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



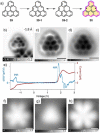

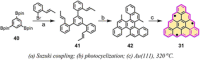

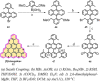
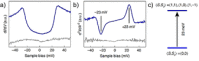



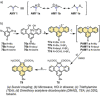
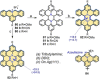
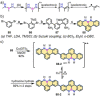
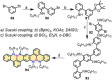



References
-
- Novoselov K. S., Geim A. K., Morozov S. V., Jiang D., Zhang Y., Dubonos S. V., Grigorieva I. V., Firsov A. A., Science 2004, 306, 666–669. - PubMed
-
- Novoselov K. S., Geim A. K., Morozov S. V., Jiang D., Katsnelson M. I., Grigorieva I. V., Dubonos S. V., Firsov A. A., Nature 2005, 438, 197–200. - PubMed
-
- Narita A., Wang X. Y., Feng X., Müllen K., Chem. Soc. Rev. 2015, 44, 6616–6643. - PubMed
-
- Diercks C. S., Yaghi O. M., Science 2017, 355, eaal1585. - PubMed
-
- Geim A. K., Novoselov K. S., Nat. Mater. 2007, 6, 183–191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

