Sacubitril/valsartan vs. angiotensin receptor inhibition in heart failure: a real-world study in Taiwan
- PMID: 32720478
- PMCID: PMC7524065
- DOI: 10.1002/ehf2.12924
Sacubitril/valsartan vs. angiotensin receptor inhibition in heart failure: a real-world study in Taiwan
Abstract
Aims: This study aimed to compare the efficacy of angiotensin receptor-neprilysin inhibitor (ARNI) therapy with angiotensin receptor blocker (ARB) therapy for cardiovascular outcomes in patients with heart failure (HF) with reduced ejection fraction.
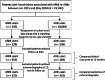
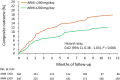
Methods and results: Data were obtained from the Chang Gung Research Database. The cohort entry date of the ARB group was assigned as that of the ARNI group to avoid immortal time bias. Additionally, 1:1 propensity score matching based on age, sex, and baseline left ventricular ejection fraction was conducted. The expectation-maximization imputation method with inverse probability of treatment weighting was used to compare outcomes between the two groups. The primary outcome was a composite of cardiovascular death and hospitalization for worsening HF. Patients who received ARNI therapy had a significantly lower risk of the primary composite outcome occurring than patients who received ARBs (hazard ratio, 0.74; 95% confidence interval, 0.57-0.96). The reduction of hospitalization for worsening HF contributed most to the primary outcome benefits. In addition to the primary outcome, the ARNI group had a significantly lower risk of non-fatal myocardial infarction. The improvement of ejection fraction was not significantly different between the groups. The medication doses of ARNI were lower than in clinical trials.
Conclusions: In patients with HF with reduced ejection fraction, sacubitril/valsartan was superior to ARB therapy in reducing the occurrence of the primary outcome endpoint of hospitalization for worsening HF and cardiovascular death.
Keywords: Angiotensin receptor blockers; Heart failure; Sacubitril/valsartan (LCZ696).
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Influence of Ejection Fraction on Outcomes and Efficacy of Sacubitril/Valsartan (LCZ696) in Heart Failure with Reduced Ejection Fraction: The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Trial.Circ Heart Fail. 2016 Mar;9(3):e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744. Circ Heart Fail. 2016. PMID: 26915374 Clinical Trial.
-
Cardiovascular and Renal Outcomes of Mineralocorticoid Receptor Antagonist Use in PARAGON-HF.JACC Heart Fail. 2021 Jan;9(1):13-24. doi: 10.1016/j.jchf.2020.08.014. Epub 2020 Nov 11. JACC Heart Fail. 2021. PMID: 33189633
-
Comparative Effectiveness of Sacubitril-Valsartan Versus ACE/ARB Therapy in Heart Failure With Reduced Ejection Fraction.JACC Heart Fail. 2020 Jan;8(1):43-54. doi: 10.1016/j.jchf.2019.08.003. Epub 2019 Dec 11. JACC Heart Fail. 2020. PMID: 31838035 Free PMC article.
-
Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial.JACC Heart Fail. 2017 Jul;5(7):471-482. doi: 10.1016/j.jchf.2017.04.013. Epub 2017 Jun 26. JACC Heart Fail. 2017. PMID: 28662936 Review.
-
The Sacubitril/Valsartan, a First-in-Class, Angiotensin Receptor Neprilysin Inhibitor (ARNI): Potential Uses in Hypertension, Heart Failure, and Beyond.Curr Cardiol Rep. 2018 Jan 27;20(1):5. doi: 10.1007/s11886-018-0944-4. Curr Cardiol Rep. 2018. PMID: 29374807 Review.
Cited by
-
Real-world experience of angiotensin receptor/neprilysin inhibitor (ARNI) usage in Thailand: a single-center, retrospective analysis.BMC Cardiovasc Disord. 2021 Jul 2;21(1):324. doi: 10.1186/s12872-021-02145-9. BMC Cardiovasc Disord. 2021. PMID: 34215190 Free PMC article.
-
ARNI in HFrEF-One-Centre Experience in the Era before the 2021 ESC HF Recommendations.Int J Environ Res Public Health. 2022 Feb 13;19(4):2089. doi: 10.3390/ijerph19042089. Int J Environ Res Public Health. 2022. PMID: 35206278 Free PMC article.
-
Angiotensin Receptor-Neprilysin Inhibitors in Patients With Heart Failure With Reduced Ejection Fraction and Advanced Chronic Kidney Disease: A Retrospective Multi-Institutional Study.Front Cardiovasc Med. 2022 Mar 8;9:794707. doi: 10.3389/fcvm.2022.794707. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35360037 Free PMC article.
-
Different Data Mining Approaches Based Medical Text Data.J Healthc Eng. 2021 Dec 6;2021:1285167. doi: 10.1155/2021/1285167. eCollection 2021. J Healthc Eng. 2021. PMID: 34912530 Free PMC article. Review.
-
Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials.ESC Heart Fail. 2022 Dec;9(6):3737-3750. doi: 10.1002/ehf2.14097. Epub 2022 Aug 3. ESC Heart Fail. 2022. PMID: 35921043 Free PMC article. Review.
References
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. - PubMed
-
- Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, Investigators C, Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet 2003; 362: 759–766. - PubMed
-
- Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, Poole‐Wilson PA, Investigators H. Effects of high‐dose versus low‐dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double‐blind trial. Lancet 2009; 374: 1840–1848. - PubMed
-
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial I. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

