Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat
- PMID: 32720646
- PMCID: PMC7398661
- DOI: 10.7554/eLife.55909
Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat
Abstract
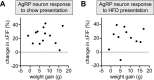
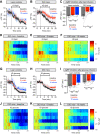
Body weight is regulated by interoceptive neural circuits that track energy need, but how the activity of these circuits is altered in obesity remains poorly understood. Here we describe the in vivo dynamics of hunger-promoting AgRP neurons during the development of diet-induced obesity in mice. We show that high-fat diet attenuates the response of AgRP neurons to an array of nutritionally-relevant stimuli including food cues, intragastric nutrients, cholecystokinin and ghrelin. These alterations are specific to dietary fat but not carbohydrate or protein. Subsequent weight loss restores the responsiveness of AgRP neurons to exterosensory cues but fails to rescue their sensitivity to gastrointestinal hormones or nutrients. These findings reveal that obesity triggers broad dysregulation of hypothalamic hunger neurons that is incompletely reversed by weight loss and may contribute to the difficulty of maintaining a reduced weight.
Keywords: homeostasis; hunger; hypothalamus; mouse; neuroscience.
© 2020, Beutler et al.
Conflict of interest statement
LB, TC, JA, SK, WS, YC, ZK No competing interests declared
Figures



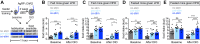
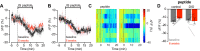

Comment in
-
Long-lasting effects of obesity on appetite neurons.Nat Rev Endocrinol. 2020 Oct;16(10):538. doi: 10.1038/s41574-020-00407-8. Nat Rev Endocrinol. 2020. PMID: 32812006 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

