Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells
- PMID: 32721127
- PMCID: PMC7941534
- DOI: 10.1002/cnr2.1133
Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells
Abstract
Background: Curcumin is a natural derivative, which exhibits broad spectrum biological activities including anti-oxidant, anti-inflammatory, and anti-cancer. Since ancient times, it has been used for the treatment of various diseases. Many reports highlighted its potential as a chemopreventive and chemotherapeutic agent. Despite its imperative properties, the pharmacological application had been limited due to low solubility in the aqueous medium, limited tissue absorption, and rapid degradation at physiological pH.
Aims: Cytotoxicity of drugs and their undesirable side effects are major obstacles in the regimens of cancer therapy. Therefore, natural plant derivatives-based anti-cancer drug delivery systems are getting more attention as they are less toxic, safer, and effective. In the present study, Pluronic block copolymer encapsulated curcumin was developed as an improved curcumin delivery system with the aim to improve its efficacy and biological response against cancer cells.
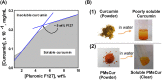
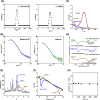
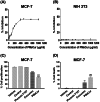
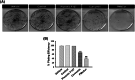
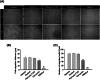
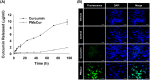
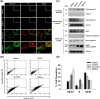
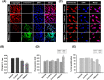
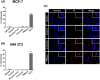
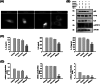
Methods and results: Pluronic micelles encapsulated curcumin was synthesized, and its characterization was done by particle size analysis, Fourier transform infrared spectroscopy, small-angle neutron scattering analysis, PXRD, and differential scanning calorimetry. Further, its biological activities were corroborated in cancer cells. Results indicate that Pluronic micelles encapsulated curcumin exemplify solubility and stability of curcumin in the aqueous medium. Biophysical characterization indicated that Pluronic F127 forms nanoparticle, and its micellar core radius was increased after incorporation of curcumin. Furthermore, biological studies show that Pluronic micelles encapsulated curcumin inhibits cell proliferation, improves cellular uptake of curcumin, arrests the cell cycle in G0/G1 phase, and inhibits the activation of NF-kB and release of pro-inflammatory cytokines to manifest apoptotic cell death rather than necrotic. This formulation was non-toxic to normal cells.
Conclusion: This study suggests that Pluronic micelles encapsulated curcumin is stable that can effectively inhibit cell proliferation and release of pro-inflammatory cytokines in cancer cells as compared with the free curcumin. This approach could be applied to improve the therapeutic index of anti-cancer agents.
Keywords: Pluronic F127; apoptosis; aqueous soluble Pluronic micelles encapsulated curcumin; cancer; curcumin.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
None declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

