Sizing, stabilising, and cloning repeat-expansions for gene targeting constructs
- PMID: 32721467
- PMCID: PMC8215685
- DOI: 10.1016/j.ymeth.2020.07.007
Sizing, stabilising, and cloning repeat-expansions for gene targeting constructs
Abstract
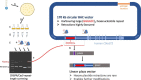
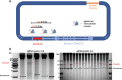
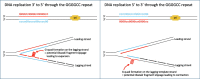
Aberrant microsatellite repeat-expansions at specific loci within the human genome cause several distinct, heritable, and predominantly neurological, disorders. Creating models for these diseases poses a challenge, due to the instability of such repeats in bacterial vectors, especially with large repeat expansions. Designing constructs for more precise genome engineering projects, such as engineering knock-in mice, proves a greater challenge still, since these unstable repeats require numerous cloning steps in order to introduce homology arms or selection cassettes. Here, we report our efforts to clone a large hexanucleotide repeat in the C9orf72 gene, originating from within a BAC construct, derived from a C9orf72-ALS patient. We provide detailed methods for efficient repeat sizing and growth conditions in bacteria to facilitate repeat retention during growth and sub-culturing. We report that sub-cloning into a linear vector dramatically improves stability, but is dependent on the relative orientation of DNA replication through the repeat, consistent with previous studies. We envisage the findings presented here provide a relatively straightforward route to maintaining large-range microsatellite repeat-expansions, for efficient cloning into vectors.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

