Succession of Bifidobacterium longum Strains in Response to a Changing Early Life Nutritional Environment Reveals Dietary Substrate Adaptations
- PMID: 32721872
- PMCID: PMC7390879
- DOI: 10.1016/j.isci.2020.101368
Succession of Bifidobacterium longum Strains in Response to a Changing Early Life Nutritional Environment Reveals Dietary Substrate Adaptations
Abstract
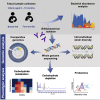
Diet-microbe interactions play a crucial role in modulation of the early life microbiota and infant health. Bifidobacterium dominates the breast-fed infant gut and may persist in individuals during transition from a milk-based to a more diversified diet. Here, we investigated adaptation of Bifidobacterium longum to the changing nutritional environment. Genomic characterization of 75 strains isolated from nine either exclusively breast- or formula-fed (pre-weaning) infants in their first 18 months revealed subspecies- and strain-specific intra-individual genomic diversity with respect to carbohydrate metabolism, which corresponded to different dietary stages. Complementary phenotypic studies indicated strain-specific differences in utilization of human milk oligosaccharides and plant carbohydrates, whereas proteomic profiling identified gene clusters involved in metabolism of selected carbohydrates. Our results indicate a strong link between infant diet and B. longum diversity and provide additional insights into possible competitive advantage mechanisms of this Bifidobacterium species and its persistence in a single host.
Keywords: Dietary Supplement; Microbiology; Microbiome.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures






References
-
- Backhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:852. - PubMed
Grants and funding
- BBS/E/F/00044409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L01632X/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

