The Role of GPR120 Receptor in Essential Fatty Acids Metabolism in Schizophrenia
- PMID: 32722017
- PMCID: PMC7459811
- DOI: 10.3390/biomedicines8080243
The Role of GPR120 Receptor in Essential Fatty Acids Metabolism in Schizophrenia
Abstract
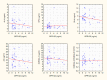
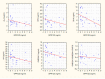
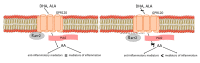
A growing body of evidence confirms abnormal fatty acid (FAs) metabolism in the pathophysiology of schizophrenia. Omega-3 polyunsaturated fatty acids (PUFAs) are endogenous ligands of the G protein-coupled receptors, which have anti-inflammatory properties and are a therapeutic target in many diseases. No clinical studies are concerned with the role of the GPR120 signaling pathway in schizophrenia. The aim of the study was to determine the differences in PUFA nutritional status and metabolism between patients with schizophrenia (SZ group) and healthy individuals (HC group). The study included 80 participants (40 in the SZ group, 40 in the HC group). There were no differences in serum GPR120 and PUFA concentrations and PUFA intake between the examined groups. In the HC group, there was a relationship between FAs in serum and GPR120 concentration (p < 0.05): α-linolenic acid (ALA) (R = -0.46), docosahexaenoic acid (DHA) (R = -0.54), omega-3 PUFAs (R = -0.41), arachidonic acid (AA) (R = -0.44). In the SZ group, FA serum concentration was not related to GPR120 (p > 0.05). In the HC group, ALA and DHA serum concentrations were independently associated with GPR120 (p < 0.05) in the model adjusted for eicosapentaenoic acid (EPA) and accounted for 38.59% of GPR120 variability (p < 0.05). Our results indicate different metabolisms of FAs in schizophrenia. It is possible that the diminished anti-inflammatory response could be a component connecting GPR120 insensitivity with schizophrenia.
Keywords: FFAR4; G protein-coupled receptors; GPR120; long-chain fatty acids; nutritional psychiatry; omega-3; polyunsaturated fatty acids; schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells.Lipids Health Dis. 2013 Jul 13;12:101. doi: 10.1186/1476-511X-12-101. Lipids Health Dis. 2013. PMID: 23849180 Free PMC article.
-
Low unesterified:esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment.Bipolar Disord. 2015 Nov;17(7):729-42. doi: 10.1111/bdi.12337. Epub 2015 Oct 1. Bipolar Disord. 2015. PMID: 26424416 Free PMC article.
-
Fish oil and flax seed oil supplemented diets increase FFAR4 expression in the rat colon.Inflamm Res. 2015 Oct;64(10):809-815. doi: 10.1007/s00011-015-0864-3. Epub 2015 Aug 15. Inflamm Res. 2015. PMID: 26275932 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4.J Mol Cell Cardiol. 2017 Feb;103:74-92. doi: 10.1016/j.yjmcc.2016.12.003. Epub 2016 Dec 14. J Mol Cell Cardiol. 2017. PMID: 27986444 Free PMC article. Review.
Cited by
-
The Role of Alpha-Linolenic Acid and Other Polyunsaturated Fatty Acids in Mental Health: A Narrative Review.Int J Mol Sci. 2024 Nov 20;25(22):12479. doi: 10.3390/ijms252212479. Int J Mol Sci. 2024. PMID: 39596544 Free PMC article. Review.
-
Corpus Callosum Microstructural Tract Integrity Relates to Longer Emotion Recognition Reaction Time in People with Schizophrenia.Brain Sci. 2022 Sep 8;12(9):1208. doi: 10.3390/brainsci12091208. Brain Sci. 2022. PMID: 36138944 Free PMC article.
-
Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia.Molecules. 2022 Dec 31;28(1):324. doi: 10.3390/molecules28010324. Molecules. 2022. PMID: 36615518 Free PMC article. Review.
-
Optimization of Neurite Tracing and Further Characterization of Human Monocyte-Derived-Neuronal-like Cells.Brain Sci. 2021 Oct 20;11(11):1372. doi: 10.3390/brainsci11111372. Brain Sci. 2021. PMID: 34827371 Free PMC article.
-
Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway.Int J Mol Sci. 2020 Dec 8;21(24):9338. doi: 10.3390/ijms21249338. Int J Mol Sci. 2020. PMID: 33302404 Free PMC article. Review.
References
-
- Clari R., McNamara R.K., Szeszko P.R. Omega-3 Polyunsaturated Fatty Acids and Antioxidants for the Treatment of Schizophrenia: A Role for Magnetic Resonance Imaging. In: Kubicki M., Shenton M.E., editors. Neuroimaging in Schizophrenia. Springer; Cham, Switzerland: 2020. pp. 367–383.
-
- De Almeida V., Alexandrino G.L., Aquino A., Gomes A.F., Murgu M., Dobrowolny H., Guest P.C., Steiner J., Martins-de-Souza D. Changes in the blood plasma lipidome associated with effective or poor response to atypical antipsychotic treatments in schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;101:109945. doi: 10.1016/j.pnpbp.2020.109945. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

