Phenylselanyl Group Incorporation for "Glutathione Peroxidase-Like" Activity Modulation
- PMID: 32722043
- PMCID: PMC7435675
- DOI: 10.3390/molecules25153354
Phenylselanyl Group Incorporation for "Glutathione Peroxidase-Like" Activity Modulation
Abstract
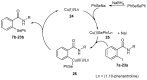
The ability of organoselenium molecules to mimic the activity of the antioxidant selenoenzyme glutathione peroxidase (GPx) allows for their use as antioxidant or prooxidant modulators in several diseases associated with the disruption of the cell redox homeostasis. Current drug design in the field is partially based on specific modifications of the known Se-therapeutics aimed at achieving more selective bioactivity towards particular drug targets, accompanied by low toxicity as the therapeutic window for organoselenium compounds tends to be very narrow. Herein, we present a new group of Se-based antioxidants, structurally derived from the well-known group of GPx mimics-benzisoselenazol-3(2H)-ones. A series of N-substituted unsymmetrical phenylselenides with an o-amido function has been obtained by a newly developed procedure: a copper-catalyzed nucleophilic substitution by a Se-reagent formed in situ from diphenyl diselenide and sodium borohydride. All derivatives were tested as antioxidants and anticancer agents towards breast (MCF-7) and leukemia (HL-60) cancer cell lines. The highest H2O2-scavenging potential was observed for N-(3-methylbutyl)-2-(phenylselanyl)benzamide. The best antiproliferative activity was found for (-)-N-(1S,2R,4R)-menthyl-2-(phenylselanyl)benzamide (HL-60) and ((-)-N-(1S,2R,3S,6R)-(2-caranyl))benzamide (MCF-7). The structure-activity correlations, including the differences in reactivity of the obtained phenyl selenides and corresponding benzisoselenazol-3(2H)-ones, were performed.
Keywords: anticancer activity; antioxidant activity; selenides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Polêto M.D., Rusu V.H., Grisci B.I., Dorn M., Lins R.D., Verli H. Aromatic Rings Commonly Used in Medicinal Chemistry: Force Fields Comparison and Interactions with Water Toward the Design of New Chemical Entities. Front. Pharmacol. 2018;9:395–414. doi: 10.3389/fphar.2018.00395. - DOI - PMC - PubMed
-
- Pacuła A.J., Mangiavacchi F., Sancineto L., Lenardao E.J., Ścianowski J., Santi C. An Update on “Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity”. Curr. Chem. Biol. 2015;9:97–112.
-
- Lenardão E.J., Santi C., Sancineto L. New Frontiers in Organoselenium Compounds. Springer International Publishing; Cham, Switzerland: 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

